Canadian Aquaculture R&D Review 2017
Finfish: Salmon
Harvest Quality Trait Evaluation to Inform Atlantic Salmon Broodstock Selection
Harvest quality traits are those that are most important to the consumer. Associated traits that are under genetic control may be incorporated into selection indices within a breeding program. Nearly all of the harvest quality traits are not appropriate for or cannot be evaluated on the breeding population itself. Instead, these traits are assessed on a commercial comparison group of breeding nucleus siblings that are raised and harvested using accepted commercial practices. Harvest evaluations present the greatest opportunity to assess multiple important traits within a single sampling effort for potential inclusion in a selective breeding program. Over 30 harvest quality traits are presently evaluated within the Atlantic Salmon broodstock program at the Huntsman Marine Science Centre. Representative traits with associated heritability, h2, for one year class include: fillet weight (0.48 ± 0.08); fillet brightness (0.39 ± 0.08); fillet redness (0.52 ± 0.09); fillet yellowness (0.55 ± 0.09); flesh moisture (0.62 ± 0.17); flesh fat content (0.60 ± 0.17); and flesh protein (0.36 ± 0.16). Additional traits assessed at this time include the omega fatty acids, other measurements of colour, maturation status, body/fillet shape, presence/absence of various deformities or abnormalities (e.g., pigmentation variation), and differential mortality/survival within commercial production environments. Each year class of the Atlantic Salmon broodstock program has an associated harvest evaluation completed that will continually build our dataset to improve selection efforts.
Date: MAY 2015–APR. 2020
Funded by: Atlantic Canada Opportunities Agency–Atlantic Innovation Fund (ACOA–AIF)
Co-funded by: New Brunswick Innovation Foundation; Northern Harvest Sea Farms Ltd.; Huntsman Marine Science Centre (HMSC)
Project Lead: Amber Garber (Huntsman Marine Science Centre)
Project Team: Chris Bridger, Susan Hodkinson, Philip Wiper, Brooke Barrett, Jamie Carpenter, Anne McCarthy, Esther Keddie, Erica Harvey, Chantal Audet (HMSC)
Collaborators: Aaron Craig (Northern Harvest Sea Farms Ltd.); Bruce Swift, Salvador Gezan (TRIGEN Fish Improvement)
Contact: Amber.Garber@huntsmanmarine.ca
Website: http://www.huntsmanmarine.ca/
Enhancing Production in Coho: Culture, Community, Catch
Coho Salmon, one of the most highly valued species in British Columbia (BC), began suffering declines in 1989 due to lower returns and high harvest rates to the point where the commercial fishery for Coho Salmon was essentially closed in 1997. Reopening the Coho Salmon fishery using recovered and enhanced populations would bring economic and social benefits to BC.
The Enhancing Production In Coho: Culture, Community, Catch (EPIC4) project aims to develop and use new genomics tools to address challenges facing safe, secure, and sustainable production of Coho Salmon. The interdisciplinary team has sequenced the Coho Salmon genome and the first results have resolved clear patterns of regional populations structuring, both within BC and at the scale of the entire distribution range. Genotyping of the hatchery broodstocks sampled in 2014 and 2015 showed strong regional population structuring and allowed high levels of accuracy in assigning salmon to specific hatcheries or geographic regions. In addition, first results on the heritability and genetic correlation shed light on the genetic basis of flesh colour and the response of this trait to artificial selection for harvest weight over the time course of eight generations. The team is also working with stakeholders, including First Nations, regarding the implementation of EPIC4 scientific knowledge about Coho Salmon to help revive and sustain the wild Coho fisheries. The work on this project could lead to more economically viable Coho Salmon fisheries serving both domestic and export markets. Our results should also be transferable to other species of Pacific Salmon as well as salmonids from other regions of Canada.
Date: OCT. 2015–SEP. 2019
Funded by: Genome Canada; Genome British Columbia
Co-Funded by: Genome Quebec; DFO; University of Victoria (UVic); Aquainnovo S.A.; Ressources Aquatiques Québec (RAQ); Institut de Biologie Intégrative et des Systemes (IBIS); Thermo Fisher Scientific Inc.
Project Leads: William Davidson (SFU); Louis Bernatchez (U Laval)
Project Team: Ben Koop, Rosemary Ommer (UVic); Roberto Neira, Jose Yanez (Universidad de Chile); Terry Beacham, Robert Devlin, Ruth Withler (DFO); Grant Murray (VIU, Duke U); Kerry Naish (U Washington); Rashid Sumaila, Ralph Matthews (UBC); Steven Jones (SFU)
Collaborators: David Willis (DFO); Brian Riddel (PSF); Jean Paul Lhorente (Aquainnovo S.A.)
Contact: ksivak@sfu.ca
Website: http://www.epic-4.org
Domestication Compromises Athleticism and Respiratory Plasticity in Response to Aerobic Exercise Training in Atlantic Salmon (Salmo salar)
In this project we address the possibility that the Norwegian Atlantic Salmon (Salmo salar) breeding program which focuses on commercially beneficial traits, such as rapid growth, may compromise the cardiorespiratory system. This may contribute to the mortality of smolts after seawater transfer.
A suite of respiratory indices (including standard metabolic rate, maximum rate of oxygen uptake, absolute aerobic scope, excess post-exercise oxygen consumption, critical oxygen level, and incipient lethal oxygen saturation) was used to evaluate aerobic capacity and hypoxia tolerance to test the hypothesis that exercise training improves the athletic robustness in both domesticated and wild strains of Atlantic Salmon. These hypotheses were tested with Atlantic Salmon parr of domesticated and wild strains that were reared under identical hatchery conditions. The two strains of fish were given either an 18-day exercise-training regime (an incremental water current of 2.0–2.8 FL s−1), or were maintained at the control water current (0.5 FL s−1) for 18 days.
While exercise training produced several tangible benefits for the wild fish, it produced very few for the domesticated fish. This shows that the domesticated strain was athletically less robust than the wild strain. These results imply that approximately ten generations of selective breeding for rapid growth in commercial aquaculture have reduced the overall athletic robustness of domesticated salmon as compared to their wild conspecifics. Given the success in improving athletic robustness of the wild strain, it still remains to be seen whether an exercise training protocol can be developed that will provide benefits to the salmon aquaculture industry.
Date: Jan. 2013–Dec. 2016
Funded by: The Research Council of Norway; The Fishery and Aquaculture Industry Research Fund
Co-Funded by: Canada Research Chairs Program (CRCP); Kone Foundation–Elizabeth R. Howland Fellowship
Project Leads: Sven Martin Jørgensen (Nofima AS); Anthony P. Farrell (UBC)
Project Team: Yangfan Zhang (UBC); Gerrit Timmerhaus (Nofima AS); Harald Takle (Marine Harvest ASA)
Collaborators: Guy Claireaux, Florian Mauduit (UBO); Katja Anttila (University of Turku); Torstein Kristensen (Nord University)
Contact: yangfan@zoology.ubc.ca
Website: http://www.zoology.ubc.ca/person/yangfan

Exercise training Atlantic Salmon (Salmo salar) in the Brett-type two-channel swimming tunnel. Photo: Yangfan Zhang (UBC)

A closer look of exercise training Atlantic Salmon (Salmo salar) in motion in the Brett-type two-channel swimming tunnel. Photo: Yangfan Zhang (UBC)
Atlantic Salmon (Salmo salar) are swimming in the Brett-type two-channel swimming tunnel. Video: Sven Martin Jørgensen (Nofima AS)
What is a Farmed Salmon? Understanding the Life of a Seafood Commodity from Ocean to Table
Farmed Atlantic Salmon is one of the world’s most valuable and widely traded seafood commodities. Its consumption is driven by increased global consumer demand that can no longer be met by wild fisheries alone. It is now the fastest growing food production system in the world. It also provides jobs and revenue for many coastal communities, including in BC where the sector is a significant economic driver in some rural, largely resource-dependent communities, including some First Nations villages. However, despite its significant economic contributions, and the ever increasing global demand for the product, both current operations and growth of the sector in BC have been consistently challenged by social license constraints (community intolerance for new and increased aquaculture development) that reflect, among other things, the deeply embedded plurality of perspectives regarding farmed salmon. Research about farmed salmon can be widely found in the ecological, economic, and business literature; however, there is a noteworthy gap in the social sciences literature.
This research uses multi-sited ethnographic methodology (observation, conversational interviews, and document analysis) to ‘follow the fish’ along the commodity chain–recording, comparing, and contrasting ideas, beliefs and knowledge about the product, both positive and negative, amongst people who engage with it on its path from production to consumption. Research sites include fish farms, processing plants, sales and distribution centres, and restaurants in Tofino, Port Hardy, Campbell River, and San Francisco.
This research will produce rich, qualitative information from employees, customers, stakeholders, and others about the production, processing, transport, culinary preparation, sales, and consumption practises for Atlantic Salmon farmed in BC. This information will increase our understanding of social-cultural issues along the entire value chain, contributing to the social license dialogue by providing greater understanding of the broader social-cultural perspectives about farmed salmon, at a time when the sector is pursuing new and increased opportunities in Canada and abroad.
Date: OCT. 2015–SEP. 2018
Funded by: MITACS Accelerate (PhD Fellowship)
Co-Funded by: Grieg Seafood BC Ltd.; Marine Harvest Canada Limited; BC Jobs Plan
Project Lead: Michele Patterson (UVic)
Project Team: Rosaline Canessa (UVic); Grant Murray (Duke U)
Collaborators: Marilyn Hutchinson (Grieg Seafood); Sharon DeDominicis (Marine Harvest Canada Limited)
Contact: patterson.michele1@gmail.com
Growth Performance of AquAdvantage® Salmon Using Two Different Diets
Attempts to enhance salmon productivity by improving diets, while maintaining low production costs, has been a challenge for the aquaculture industry. The possibility of using triploid salmon (having an extra set of chromosomes) instead of the naturally occurring diploids has increased the challenge for providing an adequate diet. AquAdvantage® salmon is a rapidly growing, all female, and triploid line of Atlantic Salmon. The objective of the current study was to examine the productivity impact of a premium diet on AquAdvantage® salmon compared to a commercial diet. For this study, 300 AquAdvantage® salmon received two different diets during two different stages of development established by weight, while 300 AquAdvantage® salmon received only a commercial Atlantic Salmon feed for the whole experimental period (~7 months). AquAdvantage® salmon fed experimental diets had significantly (P ≤ 0.05) increased thermal growth coefficient (TGC) and specific growth rate (SGR) than those fish fed the commercial feed. Results showed that the growth rate of AquAdvantage® salmon was significantly (P ≤ 0.05) improved in fish fed both starter and grower experimental diets (TGC > 2.65) as compared to the commercial feed (TGC < 2.50). At the end of the seventh month, the average weight gain difference was 184.3 g in favour of the experimental diet (P ≤ 0.05). This study suggests an opportunity to further improve growth performance in new improved diets even in a rapidly growing line. Performance of the experimental groups and effects on body composition will be discussed.
Date: MAY 2015–DEC. 2015
Funded by: National Research Council–Industrial Research Assistance Program (NRC–IRAP)
Project Lead: Dawn Runighan (Aqua Bounty Canada Inc.)
Project Team: Armando Heriazon, Christina Bullerwell (Aqua Bounty Canada Inc.); Rachid Ganga (Tyson Foods Inc.); André Dumas (Center for Aquaculture Technologies)
Contact: aheriazon@aquabounty.com
Website: https://aquabounty.com/

Difference in size of AquAdvantage® Salmon and conventional Atlantic Salmon. Photo: Berni Wood (Reel Media Studio)

Aqua Bounty Canada Inc. molecular laboratory showing the types of feed that were prepared. Photo: Berni Wood (Reel Media Studio)
Detecting Hybridization among Wild and Farmed Escaped Atlantic Salmon in Southern Newfoundland: Field Collections
The monetary value of aquaculture production has now surpassed the total value of wild fisheries. Balancing the rapid industry expansion with environmental sustainability remains a challenge, with impacts for both wild populations and industry production. Aquaculture escapees represent a continued threat to the genetic integrity of wild populations, and have been shown to interbreed with wild fish, eroding local adaptation. In southern Newfoundland, wild Atlantic Salmon populations remain at record lows and are considered ‘threatened’ by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC). Potential impacts associated with the developing aquaculture industry cannot be ruled out as a contributing factor. The aim of this study was to collect young-of-the-year Atlantic Salmon following a large (>20,000 individuals) escape event in 2013 in southern Newfoundland. This escape event was equal to or greater than the estimate of wild salmon abundance in the region. Given the magnitude of this release event, and reports of mature escapees in freshwater, these samples are expected to contain a mixture of wild and hybrid individuals. In total, 2000 juvenile Atlantic Salmon were collected. Future genomic screening of these samples will be used to quantify the rates of successful hybridization and evaluate the potential genetic impact of aquaculture escapees on wild populations in Newfoundland and Labrador.
Date: APR. 2014–MAR. 2015
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Lead: Ian Bradbury (DFO)
Project Team: Lorraine Hamilton, Patrick O’Reilly, Geoff Perry (DFO)
Contact: Ian.Bradbury@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2014-NL-02-eng.html
Genetic and Genomic Impacts of Escaped Farmed Salmon in Atlantic Canada: Evaluating the Use of Archived Atlantic Salmon Scales as a Source of Pre-Impact DNA
Aquaculture escapes are a threat to the persistence and stability of wild salmon populations; however, the presence and magnitude of these genetic impacts are difficult to quantify in practice, largely due to a lack of pre-impact genetic baseline. Historically, monitoring activities for Atlantic Salmon have collected scales for aging purposes, and these archived scales could represent a powerful source of pre-impact DNA. The main objective of this project was to explore the use of various extraction methodologies to maximize DNA yield and estimate genotyping success rate from archived Atlantic Salmon scales. Extracted DNA was quantified and used for microsatellite genotyping to demonstrate the utility of this approach. The ultimate goal was future comparison of pre- and post-aquaculture DNA samples from Atlantic Salmon in Atlantic Canada to quantify the presence and magnitude of genetic impacts due to escaped farmed salmon, thereby directly informing mitigation strategies through a quantification of impacts in space and time.
Date: APR. 2014–MAR. 2016
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Lead: Ian Bradbury (DFO)
Project Team: Lorraine Hamilton, Patrick O’Reilly, Geoff Perry (DFO)
Contact: Ian.Bradbury@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2014-NL-01-eng.html
Fundy Salmon Recovery: Recovering Endangered Atlantic Salmon through Innovation and Collaboration with Atlantic Canada’s Aquaculture Industry
Conservation sea cages–marine aquaculture sites designed to rear wild salmon–have the potential to return adults to their native rivers in numbers rivalling historic highs, provided sufficient smolts can be collected. This model could improve not only depressed numbers of wild Atlantic Salmon, but freshwater ecosystems impacted by reduced nutrient input caused by collapsed returns of diadromous fish.
The Fundy Salmon Recovery project is a collaborative approach to species recovery that includes government, nongovernment, industry, academic, and First Nation partners. Building on the success of a pilot project (2009-2012), an innovative yet practical rearing strategy has been implemented to boost endangered inner Bay of Fundy (iBoF) Atlantic Salmon populations in two native rivers. Wild smolts collected from rivers by Fundy National Park and Fort Folly First Nation are transported to Dark Harbour on Grand Manan Island, site of the world’s first wild salmon marine conservation farm. Maintained and operated by Cooke Aquaculture Inc. with support from the Atlantic Canada Fish Farmers Association, smolts are held at Dark Harbour until reaching sexual maturity. In early October, mature adults are transported to their natal rivers and released to allow for wild spawning, producing progeny free of captive exposure and associated domestication effects. Since releases of marine reared iBoF Atlantic Salmon began in 2015, over 1000 adults have been returned to the wild to spawn throughout the Upper Salmon River in Fundy National Park and the Petitcodiac River. Ongoing research through the University of New Brunswick tracks movement patterns and nutrient contributions of adults throughout the period of spawning activity.
The potential impacts of sea cage rearing are: 1) post-smolts mature in a semi-natural marine environment in the Bay of Fundy; 2) significant numbers of adults produced for release to native rivers to spawn; 3) increase in river ecosystem productivity through marine nutrient inputs; and 4) increased offspring fitness through reduced captive exposure.
Date: MAR. 2014–MAR. 2019
Funded by: Environment and Climate Change Canada (ECCC)–National Conservation Plan
Co-Funded by: Atlantic Canada Fish Farmers Association (ACFFA); Cooke Aquaculture Inc.; New Brunswick Department of Agriculture, Aquaculture and Fisheries (NBDAAF); Fort Folly First Nations; University of New Brunswick (UNB)
Project Lead: Corey Clarke (PC)
Project Team: Betty House (ACFFA); Tom Taylor (Cooke Aquaculture Inc.); Michael Beattie (NBDAAF); Tim Robinson (Fort Folly First Nations); John Whitelaw (DFO); Kurt Samways (UNB)
Contact: Corey.Clarke@pc.gc.ca
Website: http://www.fundysalmonrecovery.com/

Specially designed smolt pen utilised by the Wild Salmon Conservation Farm at Dark Harbour. Pens use a smaller mesh to accommodate smaller sized wild fish, and allow for feeding of smaller numbers of fish than typical salmon rearing operations. Photo: Nigel Fearson

Becky Graham (DFO) passes a dip net of wild exposed smolt to Garrett Momberquette (PC). These fish have been held at the DFO Mactaquac Biodiversity Facility until ready for transport to the Cooke Aquaculture Inc. operated Wild Salmon Conservation Farm at Dark Harbour on the island of Grand Manan. Photo: Nigel Fearson

Staff from Department of Fisheries and Oceans, Parks Canada Agency, University of New Brunswick, Fort Folly First Nations, and Cooke Aquaculture Inc. load wild exposed Inner Bay of Fundy Atlantic Salmon smolt into transport trucks. Staff started before dawn to make the first ferry to the island of Grand Manan where smolt will be grown at the Dark Harbour Wild Salmon Conservation Farm. Photo: Nigel Fearson
Identification of Genetic Markers Associated with Growth Performance in a Soybean Meal Based Diet for Salmon
To date, soybean meal (SBM) inclusion in salmon feed has been rather limited due to poor performance and negative physiological impacts in fish fed SBM-containing diets. However, studies have shown that there is significant individual and family variation in the ability to use SBM derived protein, and that this trait has moderately high heritability. It is thus possible to develop lines of salmon with increased efficiency in utilizing SBM. Marker assisted selection (MAS), using genetic markers such as single nucleotide polymorphisms (SNPs) associated with traits of interest, can be used to compliment traditional breeding and reduce the time required to achieve genetic gains.
This study is aimed at using genome-wide association studies (GWAS) to identify Atlantic Salmon SNPs associated with increased efficiency or tolerance to SBM as a protein source. A 60-day feeding trial was conducted to evaluate the effects of two different SBM inclusion levels (Control and Test diets with 5% and 30% SBM, respectively) on weight gain, feed efficiency, body composition, and nutrient deposition. Thirty full-sib families (initial body weight: 12.3 ± 1.0 g) of Atlantic Salmon (Saint John River strain) were utilized for the trial. Six fish per family were allocated to six different 325-liter tanks so that all families were represented equally in each tank. Each fish was tagged with passive integrated transponders (PIT) and each diet was allocated to three tanks.
The expected outcome of this project is a set of SNPs associated with increased tolerance and/or efficiency in utilizing SBM as a protein source. These novel markers will play a key role in the development of improved genetic lines that will allow for a significant increase in SBM inclusion in aquafeeds for Atlantic Salmon.
Date: MAY 2016–DEC. 2016
Funded by: Soy Aquaculture Alliance
Project Lead: Jason Stannard (CAT)
Project Team: John Buchanan (CAT); Debbie Plouffe, Tiago Hori, André Dumas (CATC)
Collaborators: Cooke Aquaculture Inc.
Contact: adumas@aquatechcenter.com
Website: http://aquatechcenter.com/
High Density SNP Linkage Map for North American Atlantic Salmon (Salmo salar)
Atlantic Salmon continue to be a commercially and ecologically important species. In regards to aquacultural practices, understanding where genes that influence commercially important traits (such as growth and disease resistance) are located on the genome as well as determining how often genes from the same chromosome are inherited together is of growing importance for improving the development of selective breeding programs. Creating genetic linkage maps and using genetic markers such as single nucleotide polymorphisms (SNPs) to trace inheritance from parents to offspring between individuals is a necessary first step before quantitative traits can be mapped onto the genome.
We used 220K and 50K SNP chip genotypes from the parents and offspring of seven large families for constructing sex-specific genetic linkage maps through the “onemap” package in R (a software and coding language commonly used for statistical computing and data analysis). The initial male and female maps for each of the 27 chromosomes were created using 11K SNP genotypes from three families of North American Atlantic Salmon (Saint John River strain). We currently aim to create consensus linkage maps that would merge individual chromosomes between families for all 45,000 SNPs that overlap both custom SNP chips. In addition to benefitting selective breeding programs, the consensus linkage maps will also be compared to the newly published physical map for European Atlantic Salmon. This will facilitate the identification of genes for commercially important traits that may differ in genomic location between geographically distinct populations.
Creating highly informative consensus maps for North American S. salar will enable selective breeding programs to identify causal loci that influence important traits such as growth or disease resistance. It will also facilitate research in salmon conservation by identifying functional genetic differences among stocks.
Date: JAN. 2016–AUG. 2018
Funded by: Genome Canada; Genome Atlantic; Ontario Genomics
Co-Funded by: NRC–Industrial Research Assistance Program (NRC–IRAP); Kelly Cove Salmon Ltd.
Project Leads: Elizabeth Boulding (U Guelph); Keng Pee Ang (Kelly Cove Salmon Ltd.)
Project Team: Melissa MacLeod-Bigley, Forest Dussault, Jane Tosh, Melissa Holborn, Larry Schaeffer (U Guelph); Jake Elliott, Frank Powell (Cooke Aquaculture Inc.)
Collaborators: Matthew Kent, Harald Grove, Sigbjørn Lien, Thomas Moen (Norwegian U Life Sciences)
Contact: boulding@uoguelph.ca
Website: https://www.uoguelph.ca/ib/boulding
Using a Genomics Approach to Identify Atlantic Salmon Aquaculture Escapees and Hybrids
The cultivation of Atlantic Salmon has increased exponentially since the late 1960s with expansion into new geographic areas (e.g., Newfoundland) and the use of new selectively bred strains from North America and Europe. Coincident with this expansion has been an increased risk of farmed salmon escapes, which has the potential to impact the diversity of wild Atlantic Salmon populations. This project attempts to identify and apply targeted groups of genetic markers to quantify the genetic impacts of farmed Atlantic Salmon on wild populations and the frequency of interbreeding in the wild. This proposal directly targets client needs and is a first step towards identifying impacts and strategies for alleviating those impacts that result from interactions between wild and farmed salmon escapees in Atlantic Canada.
This project extends earlier work that developed expertise for genome wide marker development in non-model species. In this proposal, the impact of farmed escapees on wild populations will be quantified by: 1) combining existing genomic data and modern DNA sequencing methods to develop a group of genetic markers (single nucleotide polymorphisms or SNPs) for rapid and accurate identification of farmed salmon escapees, including all strains in use, or under consideration for use, in Atlantic Canada; and 2) applying this genomic screening tool to rapidly and accurately quantify, both, the presence of escapes and recent hybrids in the wild focusing on Newfoundland and Maritimes regions.
Research has confirmed that escaped farmed salmon are breeding with wild salmon and producing offspring in many rivers in Newfoundland.
Date: APR. 2014–MAR. 2017
Funded by: DFO–Genomics Research and Development Initiative (DFO–GRDI)
Co-funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Lead: Ian Bradbury (DFO)
Project Team: Lorraine Hamilton, Patrick O'Reilly (DFO)
Collaborators: Geoff Perry, Dounia Hamoutene, Martha Robertson (DFO); Elizabeth Barlow (DFA); Jon Carr (Atlantic Salmon Federation); Ross Hinks (Aboriginal Government–Miawpukek First Nation)
Contact: Ian.Bradbury@dfo-mpo.gc.ca
Website: Using a Genomics Approach to Identify Atlantic Salmon Aquaculture Escapees and Hybrids

Sampling for possible offspring of escaped farmed Atlantic Salmon. Photo: Chris Hendry (DFO)
Use of Hydro-Acoustic Methods to Assess the Migration Timing and Distribution of Juvenile Salmon in Discovery Islands and Johnstone Strait
During their migration to the Northern Pacific, juvenile wild salmon from the Strait of Georgia pass through the Discovery Islands and Lower Johnstone Strait, where salmon farming occurs.
This project seeks to inform the risk of disease transfer associated with interactions between wild and farmed salmon in this area by studying wild salmon migratory pathways and the duration of their residency in the vicinity of these fish farms.
In conjunction with traditional survey/sampling methods (including purse seining, beach seining, and trawling) using hydro-acoustics offers a cost-effective way of monitoring fish abundance, behaviours, and distribution for extended and continuous periods of time. This will enable observation and data collection on juvenile wild salmon migration in the area of fish farms, thus gaining insights into potential impacts of wild salmon on farmed salmon and vice versa.
The results of this project will:
- Assist in informing a risk assessment process to investigate the risks of pathogen transfer from farmed to wild salmon, particularly in the Discovery Islands.
- Improve our understanding and inform and support the development of evidence-based aquaculture management approaches.
- Provide information in addressing critical uncertainties and recommendations as identified in the Cohen Report with respect to: 1) marine survival in their early marine life; 2) disease transfer and interactions between wild and farm salmon; and 3) assessing the cumulative impacts of multiple stressors on Fraser River Sockeye Salmon productivity.
- Inform operational decisions and best practices engaged in by the BC salmon aquaculture industry.
Date: MAY 2015–JUN. 2017
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: BC Salmon Farmers Association (BCSFA)
Project Lead: Stéphane Gauthier (DFO)
Project Team: Stewart Johnson, Marc Trudel, Chrys-Ellen Neville (DFO)
Collaborators: Joanne Liutkus (BCSFA)
Contact: Stephane.Gauthier@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/15-1-P-02-eng.html
The Historical and Social Dimensions of Salmon Aquaculture Science
For three decades, salmon aquaculture has been a focus of environmental research. In this project I am applying the tools of environmental history and science and technology studies to understand how this research has developed, as well as the roles it has played in public discussions regarding the industry. Several more specific objectives are also being pursued.
First, I am writing an environmental history of salmon aquaculture science. This history will explore the relations between scientific research and the evolving environmental, social, and political dimensions of the industry.
Second, I am examining how the diverse institutions engaged in environmental research–governments, universities, industry, and public interest organizations–have shaped research priorities, research results, and the application of these results.
Third, I am investigating the movement of scientific knowledge of salmon aquaculture among research sites in Canada, Norway, Ireland, and Scotland.
Fourth, I am examining the prospects for effective science that is able to contribute to help resolve controversies regarding this industry.
While this project is examining the full range of environmental science relating to salmon aquaculture, a special focus is on research relating to sea lice.
This project is providing a better understanding of how salmon aquaculture science has developed in relation to the growth of the industry in its varied environmental and social contexts.
Date: JUN. 2007–DEC. 2018
Funded by: Social Sciences and Humanities Research Council of Canada (SSHRC)
Project Lead: Stephen Bocking (Trent U)
Contact: sbocking@trentu.ca
Website: Stephen Bocking

Salmon farm near Campbell River, British Columbia. Photo: Stephen Bocking (Trent U)
Migration Timing and Distribution of Juvenile Salmon in Discovery Islands and Johnstone Strait
This research is determining how juvenile salmon utilize the Strait of Georgia, including the Discovery Islands area, with a focus on Fraser River Sockeye Salmon and to a lesser extent, Chinook Salmon. It will also provide the information required to fully assess the risks of disease transfer from salmon arms to the wild, understand the potential consequences of such transfers, and inform farm management policies.
Purse seines and DFO trawl surveys have greatly increased the understanding of the migration and health of juvenile salmon within the Strait of Georgia, BC, especially for Sockeye Salmon. Surveys conducted in 2010–2012 revealed that Fraser River Sockeye Salmon do not enter the Discovery Islands area (a fish farming area) until the end of May, and that they are widely distributed throughout this area for at least part of June. To further assess risks associated with interactions between farmed and wild fish, information in the following key areas is needed: 1) knowledge of migratory pathways of salmon and the duration of their residency in the vicinity of fish farms; 2) knowledge of the prevalence of pathogens and diseases within wild and farmed populations; and 3) knowledge of environmental and host conditions during the periods wild salmon reside in the vicinity of fish farms. Additionally, more information is required to further understand when and for how long juvenile salmon are present in the vicinity of fish farms, as well as to describe migration timing of juvenile Fraser River Sockeye Salmon out of the Strait of Georgia. To gain this required information, sampling will be performed using a three-year trawl survey in the Strait of Georgia and a three-year purse seine combined with hydroacoustic surveys in Johnstone Strait.
Date: APR. 2014–JUN. 2017
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Marine Harvest Canada Limited; Grieg Seafood BC Ltd.; Cermaq Canada Ltd.
Project Lead: Stewart Johnson (DFO)
Project Team: Marc Trudel, Chrys-Ellen Neville (DFO); Diane Morrison (Marine Harvest Canada Limited); Patrick Whittaker (Grieg Seafood BC Ltd.); Barry Milligan (Cermaq Canada)
Contact: Stewart.Johnson@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/P-14-01-001-eng.html

Discovery Islands. Photo: Dan McPhee (DFO)
Spatial and Temporal Distribution and Survival of Farmed Atlantic Salmon after Experimental Release from Sea Cage Locations
The expansion of the aquaculture industry in Newfoundland and the decline in wild salmon stocks have raised questions regarding the possible impacts escaped farmed salmon may have on local wild populations. Despite increased industry awareness and the implementation of a code of containment, escape events can still occur. Spawning between aquaculture-origin Atlantic Salmon and wild Atlantic Salmon has been scientifically documented in Newfoundland. Further research is needed to better understand the potential risk of escapees on wild salmon populations. The objective of this project is to determine the residency time, locations, migratory routes, and survival rates of escaped farmed Atlantic Salmon by monitoring the movements of acoustically-tagged smolts, post-smolts, and adults, following a simulated escape of a group of fish at different times of the year. Identifying the migratory routes followed by escapees, as well as residency patterns and how they vary with the timing of the escape event (seasonal effects), will help in designing more efficient recapture strategies.
The research results from this study will inform federal and provincial ecosystem-based management of the industry and provide key information for the development of strategies to minimize potential impacts of escaped farmed Atlantic Salmon on the environment and wild salmon populations.
Date: APR. 2014–MAR. 2017
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Lead: Dounia Hamoutene (DFO)
Project Team: Curtis Pennell, Kimberley Marshall, Dwight Drover, Sebastien Donnet, Keith Clarke, Geoff Perry (DFO)
Contact: Dounia.Hamoutene@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2014-NL-04-eng.html
Studying Effects of Atlantic Salmon Broodstock Age and Egg Size on Later Performance of Progeny
Atlantic Salmon broodstock that have not matured after four years might be culled or retained for use the following year for various reasons. For example, families of Atlantic Salmon that have a higher than average prevalence for late sexual maturation may be bred to try to delay sexual maturation within production fish. Female broodstock that mature at five years of age have significantly larger eggs (on average) compared with eggs from four-year-old females. Anecdotally, it is often believed that this larger egg size provides an advantage to the same individuals throughout the production cycle–larger eggs yield larger progeny that is carried on through to harvest. We are comparing egg sizes from all donor female broodstock and resulting growth performance of progeny at various ages at the time of measurement where female and male broodstock of different ages were crossed (four-year-old female x four-year-old male, 4 x 5, 5 x 4, and 5 x 5). These crosses are controlled within the same production cycle and at the same physical location thereby reducing and/or removing environmental variation. To date, egg size has not defined juvenile size (assessed up to a post-smolt, pre-harvest stage). With the first year of data evaluated, the largest progeny, on average, resulted from 4 x 4 year old crosses despite having the smaller egg size. It appears that the growth potential of juveniles is based on inherent genetic variation or essentially genetic growth potential rather than egg size.
Various factors are considered when retaining broodstock based on age or eggs based on average egg size from a batch. This line of research has direct commercial relevance within production or selection of broodstock even in the absence of a pedigreed broodstock program.
Date: FEB. 2016–APR. 2020
Funded by: Atlantic Canada Opportunities Agency–Atlantic Innovation Fund (ACOA–AIF)
Co-Funded by: New Brunswick Innovation Foundation; Northern Harvest Sea Farms Ltd.; Huntsman Marine Science Centre (HMSC)
Project Lead: Amber Garber (HMSC)
Project Team: Chris Bridger, Susan Hodkinson, Philip Wiper, Jamie Carpenter, Danny Craig, Esther Keddie, Brooke Barrett, Erica Harvey, Chantal Audet (HMSC)
Collaborators: Aaron Craig (Northern Harvest Sea Farms Ltd.)
Contact: Amber.Garber@huntsmanmarine.ca
Website: http://www.huntsmanmarine.ca/
Thermal and pH Tolerance of Farmed, Wild, and First Generation Farmed-Wild Hybrid Salmon
In Newfoundland and Labrador (NL), all farmed Atlantic Salmon (Salmo salar) originate from the Saint John River strain (New Brunswick). It is believed that wild stocks have developed adaptations to their local environment; therefore the vulnerability of these local, genetically distinct stocks to farmed escapees through interbreeding is a concern. Studies on interactions between wild and farmed salmon have shown that this issue is area-specific and therefore these interactions need to be further explored within Newfoundland and Labrador.
This research sought to clarify the ability of F1 hybrids (offspring of local wild and domesticated strains) to survive under local environmental conditions (i.e., reduced pH level of river waters and low spring seawater temperatures) occurring in Newfoundland and Labrador. The results of this research provide information on the potential impact of farmed escapees on wild stocks.
We found that after a 90-day exposure to low pH water, no differences were observed between pure wild parr and F1 hybrids in survival, growth, and gill enzymes indicative of seawater readiness, but pure farmed parr had lower survival than the F1 hybrids. Additionally, we found no significant differences in total mortality among wild, farmed, and hybrids after transfer to seawater and exposure to very cold temperatures.
While pure farmed salmon might be affected in river conditions, this research suggests that hybrids (most likely the outcome of farm-wild reproductive interactions) would not experience a significant mortality due to low pH in Newfoundland rivers. The findings also suggest that hybrids resulting from crossing wild salmon and farmed Saint John River salmon are as likely to survive seawater migration in cold temperatures as their wild counterparts.
Date: APR. 2014–JUN. 2015
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Cold Ocean Salmon Inc.; Northern Harvest Sea Farms Ltd.
Project Lead: Dounia Hamoutene (DFO)
Project Team: Lynn Lush, Kimberley Burt (DFO)
Collaborators: Julia Jensen (Cold Ocean Salmon Ltd.); Jennifer Caines (Northern Harvest Sea Farms Ltd.)
Contact: Dounia.Hamoutene@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/N-14-01-002-eng.html
Reduction of Ammonia and Solids from Chinook Salmon Culture Facilities
In farmed fish, metabolic processes produce nitrogenous (principally ammonia) wastes which are released into the environment. That fish are not efficiently utilizing the feed for growth and maintenance represents a potential economic loss. Additionally, the release of nitrogenous wastes into the environment can have implications for the ecosystem and the fish farm, and regulatory consequences for culture facilities.
This study explored how feed regimes designed to stimulate compensatory growth may be used during Chinook Salmon production to reduce nitrogen excretion into the environment and increase food utilization by the fish. When faced with modest periods of food deprivation, the salmon were found to maintain growth and excrete less nitrogen, despite the similarity of their final weights to the control group. This indicates that repeated short-term food deprivation of two days duration over a seven week cycle may provide a useful strategy for reducing nitrogen loss to the environment, while minimizing the loss of growth potential of the fish.
The results of this research indicate the possibility for using cyclical feeding to reduce nitrogen excretion during salmon farming. This would be especially beneficial at land-based salmon farms with systems where the water discharge is focused to a single outflow, recirculating aquaculture, and multiple net-pens or tanks.
Date: AUG. 2012–SEP. 2015
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-funded by: Agrimarine Industries Inc.
Project Lead: Ian Forster (DFO)
Project Team: Lawrence Albright (AgriMarine Industries Inc.)
Collaborators: Lawrence Albright, Robert Walker (AgriMarine Industries Inc.)
Contact: Ian.Forster@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/P-12-01-001-eng.html
Determination of the Potential Spatial Overlap and Interaction Between Commercial Fisheries (American Lobster, Snow Crab) and Finfish Aquaculture Activities in Connaigre Bay, Newfoundland
The objective of this project was to provide valuable information to inform future site development initiatives and contribute to the sustainability of the fishing and aquaculture industries on the south coast of Newfoundland and Labrador.
There is rarely an opportunity to collect and compare ecological data before, during, and after a salmon farming site has been approved and is under production. This collected environmental and biological data at two newly approved salmon aquaculture sites in Connaigre Bay, Newfoundland and Labrador–a bay that has not yet held salmon production sites. Unfortunately, the aquaculture sites were not stocked according to the expected time-frame, thus, the project was terminated prematurely. Data collected over the course of the project are currently being employed to establish habitat preferences and species distribution mapping. This can be used as a tool to advise on potential spatial overlap between aquaculture sites and local fisheries.
Date: APR. 2012–JUN. 2017
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-funded by: Fish, Food, and Allied Workers Union (FFAW); Cold Ocean Salmon Inc.
Project Lead: Dounia Hamoutene, Pierre Goulet (DFO)
Project Team: Andry Ratsimandresy, Dwight Drover, Darrell Mullowney, Don Stansbury (DFO); Jeff Barrell, Jon Grant (Dalhousie U)
Collaborators: Harvey Jarvis (FFAW); Jennifer Woodland (Cold Ocean Salmon Inc.)
Contact: Dounia.Hamoutene@dfo-mpo.gc.ca, Pierre.Goulet@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/N-12-02-001-eng.html
Family Variation and Heritability of Male and Female Atlantic Salmon Fitness Traits
There is minimal information published associated with the heritability of male or female Atlantic Salmon broodstock fitness traits. Documenting relevant data requires tracking individuals from known families through maturation and gamete collection. During the 2014 and 2015 spawning seasons of mature Atlantic Salmon, 1056 milt samples were assessed from 529 males and egg samples were assessed from 1139 females. These broodstock originated from 130 different families over two year classes with various levels of relatedness among families. Heritability and genetic correlations were assessed considering various traits including: for both males and females total body weight (kg) and gamete volume (mL); for males only sperm density, milt volume from repeated stripping (repeatability); and for females only egg size. From collected data, fecundity and total number of gametes by individual were calculated. Data continues to be collected with each new spawning season. In addition, effect of age (primarily four and five year old broodstock) and photo-thermal manipulation of spawning time on male and female gamete production related traits is under evaluation.
Knowledge of fitness traits and heritability may inform decisions within a broodstock program related to selection, numbers of each sex to maintain, and expected number of eggs resulting from the Atlantic Salmon retained for spawning.
Date: NOV. 2014–APR. 2020
Funded by: Atlantic Canada Opportunities Agency–Atlantic Innovation Fund (ACOA–AIF)
Co-Funded by: New Brunswick Innovation Foundation; Northern Harvest Sea Farms Ltd.; Huntsman Marine Science Centre (HMSC)
Project Lead: Amber Garber (HMSC)
Project Team: Chris Bridger, Susan Hodkinson, Philip Wiper, Jamie Carpenter, Danny Craig, Esther Keddie, Brooke Barrett, Erica Harvey, Chantal Audet (HMSC)
Collaborators: Aaron Craig (Northern Harvest Sea Farms Ltd.)
Contact: Amber.Garber@huntsmanmarine.ca
Website: http://www.huntsmanmarine.ca/
Hybridization of Farmed Escaped and Wild Atlantic Salmon: So What? An Empirical and Model Based Exploration of the Consequences for Wild Populations throughout the North Atlantic
The farming of Atlantic Salmon now exceeds two million tonnes worldwide which exceeds the natural production of wild populations. Interbreeding between wild and escaped farmed salmon has been reported both in Europe and North America and can alter wild population characteristics, eroding local adaptation and causing wild population declines. However, the extent and magnitude of these genetic impacts are difficult to predict. The resiliency of wild populations, the recovery time following hybridization, and the efficacy of possible mitigation strategies remain unclear.
The overall goal of this international collaborative research project is to provide the basis for robust scientific advice regarding the genetic impacts of escaped farmed Atlantic Salmon on wild populations both locally (i.e., Newfoundland) and across the North Atlantic. As well, potential successes of various mitigation strategies will be explored by using data collected in parallel research projects from across the North Atlantic. This work will directly complement existing studies and will: 1) quantify the magnitude of hybridization between wild and escaped farmed salmon and explore growth, survival, and biological differences between wild and hybrid individuals; and 2) develop an international collaborative project focused on evaluating different models used throughout the North Atlantic.
Identifying risks and potential mitigation strategies associated with Atlantic Salmon aquaculture escapees is necessary for the successful conservation of wild salmon populations, the stability of recreational and Aboriginal fisheries, and continued growth of a sustainable aquaculture industry. Through an examination of the presence of interbreeding and introgression, this work will help identify strategies for maintaining the rapid growth of the aquaculture industry without altering population structure or local adaptation of wild salmon populations. By doing so, this work will directly assist DFO in meeting Canada’s commitment to ensure the aquaculture industry develops sustainably.
Date: MAR. 2016–MAR. 2019
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Lead: Ian Bradbury (DFO)
Collaborators: Ian Flemming, Matt Rise (MUN); Kjetil Hindar (Norwegian Institute for Nature Research); Kevin Glover (Institute of Marine Research, Norway); Mark Coulson, Eric Verspoor (The Rivers and Lochs Institute, Scotland); Phil McGinnity (School of Biological, Earth, and Environmental Sciences, Ireland); Einar Nielsen (Technical University of Denmark); Kristen Gruenthal (NOAA Fisheries)
Contact: Ian.Bradbury@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2016-NL-11-eng.html
Quantifying Direct Genetic Impacts of Escaped Farmed Salmon on Wild Salmon in Atlantic Canada
Aquaculture escapes are a threat to the persistence and stability of wild salmon populations, with impacts occurring through both genetic and ecological interactions. The goal of this study is to quantify the presence and magnitude of direct genetic impacts that escaped farmed salmon have on wild salmon populations in order to inform management decisions and advise on mitigation strategies. Specifically, this study addresses three objectives: 1) to quantify the magnitude of low level chronic escapes through an annual targeted survey; 2) to quantify annual variation in hybridization among wild and farm escaped Atlantic Salmon; and 3) to evaluate at sea survival of hybrids in Newfoundland.
Identifying risks and potential mitigation strategies associated with Atlantic Salmon aquaculture escapees is critical to both the successful continued growth of the aquaculture industry and the conservation of wild salmon populations.
This study will begin to quantify the extent of genetic impacts from farm escaped Atlantic Salmon on wild populations over time and in different areas in Atlantic Canada.
Date: APR. 2016–MAR. 2019
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Lead: Ian Bradbury (DFO)
Project Team: Lorraine Hamilton, Carole Grant, Chris Hendry, Brian Dempson, Ross Jones (DFO)
Collaborators: Ian Fleming (MUN); Jon Carr (Atlantic Salmon Federation); Ross Hinks (Miawpukek First Nation); Tony Blanchard, Derek Tobin, Gerald Cline, Lloyd Slaney, Helen Griffiths (DFO)
Contact: Ian.Bradbury@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2016-NL-02-eng.html
Probability of Detecting Escaped Aquaculture Salmon is Related to Distance Between Production Areas and Rivers
Salmon that escaped from sea cage aquaculture facilities have been detected in a large number of rivers in Eastern North America with the detection of escapees seemingly related to the distance of the river from the production areas. Morris et al. (2008) compiled the existing information available to 2007 on aquaculture escapees in rivers of Eastern North America but did not provide any quantitative analysis that could be used to link the detection of escapees relative to the distance of the rivers from the production areas. This project proposes to update the compilation of observations from Morris et al. (2008) to 2015, to review the literature on the behaviour of escaped salmon, and based on these components, to examine a series of models with differing assumptions on behaviour and diffusion, for their ability to generate the empirical observations of escapees in monitored rivers. The objective is to examine models that would provide probability statements of observing an escaped salmon in a river based on the distance of the river from the escape location, the intensity of the escape event, and the river monitoring effort. This knowledge will inform aquaculture management siting decisions.
Reference: Morris, MRJ., Fraser, D.J., Heggelin, A.J., Whoriskey, F.G., Carr, J.W., O’Neil, S.F., and Hutchings, J.A. 2008. Prevalence and recurrence of escaped farmed Atlantic Salmon (Salmo salar) in Eastern North American rivers. Can. J. Fish. Aquat. Sci. Vol. 65: 2807-2826.
Date: MAY 2016–MAR. 2017
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Lead: Ian Bradbury (DFO)
Project Team: Brian Dempson, Ross Jones, Alex Levy (DFO)
Contact: Ian.Bradbury@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2016-NL-14-eng.html
Heritability of Blood Parameters as Health Indices for an Atlantic Salmon (Salmo salar) Selective Breeding Program
The Atlantic Salmon broodstock program at the Huntsman Marine Science Centre (HMSC), with commercial partner Northern Harvest Sea Farms Ltd., is selecting for traits that are important to commercial production. Many of these traits cannot be measured directly on breeding candidates but have to be recorded on close relatives (e.g., progeny from the same families), thereby selecting based on performance of the family versus the individual. One limitation of this approach is that it is possible to select an individual that does not possess enhanced performance for the desired trait (e.g., disease resistance/susceptibility). Use of genomic markers in a broodstock program mitigates this issue after the molecular markers are demonstrated to be reliable for predictive purposes. Alternatively, biological proxy indicators may be discovered that are readily measurable and closely correlate with specific commercial traits of interest to increase selection efficiency as well. As an example, during the 2016 spawning season, 1217 blood samples were collected from individual male and female broodstock representing 195 families from three ages, multiple rearing conditions, and at various points during and after typical spawning. Blood parameters recorded from samples included lymphocyte and neutrophil density, erythrocyte size, and hematocrit. Heritability of these parameters, their reliability as health indicators, and correlations among these traits and other commercial traits are under evaluation. Preliminary analysis indicates significant differences in hematocrit among broodstock reared under ambient conditions compared to those receiving photo-thermal-advanced conditions.
The encouraging preliminary research complements the extensive data array for the Atlantic Salmon broodstock program and may provide another monitoring tool in the production of salmon.
Date: OCT. 2016–DEC. 2020
Funded by: Atlantic Canada Opportunities Agency–Atlantic Innovation Fund (ACOA–AIF)
Co-Funded by: New Brunswick Innovation Foundation; Northern Harvest Sea Farms Ltd.; Huntsman Marine Science Centre (HMSC)
Project Leads: Duane Barker, Amber Garber (HMSC)
Project Team: Anne McCarthy, Chris Bridger, Susan Hodkinson, Esther Keddie, Rebecca Eldridge, Erica Harvey, Ellen Fanning, Chantal Audet, Brooke Barrett (HMSC)
Collaborators: Aaron Craig (Northern Harvest Sea Farms Ltd.)
Contact: Duane.Barker@huntsmanmarine.ca
Website: http://www.huntsmanmarine.ca/
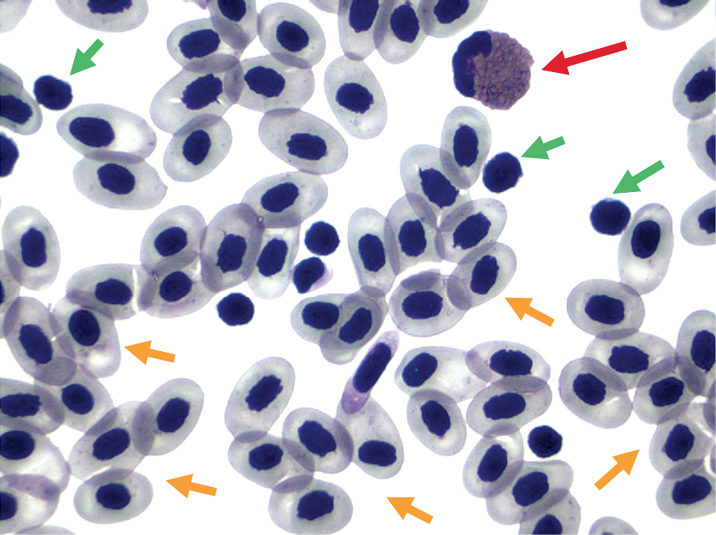
Fish blood (stained with Diff-Quik™) illustrating predominantly erythrocytes (orange arrows), with few lymphocytes (green arrows) and one neutrophil (red arrow). Image captured at 1000X. Photo: Duane Barker (HMSC)
- Date modified:

