Canadian Aquaculture R&D Review 2017
Sea Lice
Characterizing the Mechanism of Salmon Louse (Lepeophtheirus salmonis) Rejection by Coho Salmon (Oncorhynchus kisutch) Using a Novel Dual RNA-Sequencing Approach
After infection with larval sea lice (Lepeophtheirus salmonis), juvenile Coho Salmon are able to rapidly reject the parasite. This rejection is associated with aggressive hyperplasia and infiltration of cellular effectors; however, the molecular mechanisms of this response have not been characterized. This project aims to explore the response of both salmon and sea louse simultaneously by leveraging high-throughput modern sequencing technologies (dual RNA-seq). Samples of Coho Salmon fins with and without visible sea lice infestation were used to generate a pool of transcripts originating from both the sea lice and the salmon. Reads arising from either organism were separated using publicly available transcriptome/genome information for both species. Differential expression was analyzed for both the host and the parasite comparing different time-points throughout the progression of the infection. Several pathways of the immune response were shown to be activated in Coho Salmon such as the complement cascade, tissue remodelling, and an acute phase response, while a concomitant response in the sea louse featured activated oxidative stress and cell death pathways. The analysis also revealed that mRNA expression reached basal levels after 18 days post-infection, indicating that rejection mechanisms were most likely completed by that time. To our knowledge, this is the first time the transcript expression of both host and parasite has been simultaneously investigated in salmonids using RNA-seq.
The results of this project demonstrates the feasibility of dual RNA-seq while also putting forward many hypotheses related to the molecular mechanism behind sea lice resistance.
Date: DEC. 2015–DEC. 2017
Funded by: Natural Sciences and Engineering Research Council (NSERC)–Postdoctoral Fellowship Program
Co-Funded by: Elanco Animal Health
Project Lead: Mark Fast (UPEI–AVC)
Project Team: Laura Braden (UPEI–AVC); Tiago Hori (CAT); Phil Byrne (DFO)
Contact: lbraden@upei.ca

Sea lice are encapsulated by an aggressive host response in the skin (right) and fin (left) of Coho Salmon (shown here at 6 days post-infection). Photo: Laura Braden (UPEI-AVC)

A micrograph (H&E, 200X magnification) showing a sea louse attached to Coho Salmon fin, with massive recruitment of cellular responders (small round blue cells) below attachment site at four days post-infection.
The Use of Kelp Perch and Pile Perch to Control Sea Lice (Lepeophtheirus salmonis) on Infested Atlantic Salmon Smolts
Sea lice infestations in farmed Atlantic Salmon require regular monitoring and, sometimes, treatments with chemicals such as SLICE®. Alternative methods to control sea lice infestations are needed to improve the sustainability of Atlantic Salmon farming. Biological controls as a method to control sea lice infestations have been considered as viable mitigation tools to reduce sea lice infestations for many years and are now being successfully used in Norway and Scotland, with cleaner fish such as wrasse and lumpfish.
The goal of this research was to demonstrate if Kelp Perch (Brachyistius frenatus) and Pile Perch (Rhacochilus vacca) would clean sea lice off infested Atlantic Salmon. Several cohabitation trials have been performed whereby Kelp or Pile Perch were placed in tanks with sea lice infested salmon. While variation in feeding activity exists, perch of both species actively cleaned infested salmon of parasitic sea lice within 48 hours of cohabitation. Video evidence supports this result showing perch actively feeding on parasitic sea lice. Additionally, digested sea lice were found within cohabitating perch gastrointestinal tracts and in fecal casts recovered from experimental tanks. Salmon body condition and health showed no observable differences between experimental and control tanks, indicating that active sea lice predation by perch does not result in negative health impacts to infested salmon.
This research addresses a large issue facing the salmon farming industry in BC by conducting research to find a sustainable way to reduce the environmental, economic, and public perception issues related to sea lice infestations in farm raised Atlantic Salmon. The use of cleaner fish to reduce sea lice infestations is an environmentally friendly solution to this issue of protecting wild salmon populations.
Date: DEC. 2014–ONGOING
Funded by: Sea Pact; BCSFA–Marine Environment Research Program (BCSFA–MERP)
Co-Funded by: Marine Harvest Canada Ltd.
Project Lead: Shannon Balfry (Vancouver Aquarium)
Project Team: Maureen Finn (Living Elements Ltd.); Sam Ferguson, Selina Thorberg (Vancouver Aquarium)
Collaborators: Diane Morrison (Marine Harvest Canada Ltd.); Simon Jones (DFO)
Contact: Shannon.Balfry@vanaqua.org
Kelp Perch (Brachyistius frenatus) cleaning sea lice off of an Atlantic Salmon (Salmo salar) smolt. (A) Kelp Perch approaching the left side of the tail of an Atlantic Salmon. (B) The instant the Kelp Perch picks a sea louse off of the salmon, notice the jaw protrusion. (C) and (D) The moments after the pick as the perch begins to retreat from the salmon. Photo: Sam Ferguson (Vancouver Aquarium)
Wild Salmon Sea Lice Data Integration–Sea Lice Monitoring Network Development for Wild Salmon in Vancouver Island Coastal Waters
Sea lice monitoring programs on wild Pacific smolts have served to broaden the understanding of sea lice ecology and help to further develop sea lice management protocols on salmon farms. Studies where a large amount of annual data has been collected present a novel opportunity to study patterns over time, particularly where such studies offer structured snap-shots in space and/or time. To fully capture the value of these types of studies, it is essential that they be integrated for the purposes of comparison and aggregation.
This program aims to create a single repository for sea lice monitoring data from wild fish in British Columbia (BC), collected by the salmon farming industry, and supplemented by additional data sets generated from conservation groups, academics, First Nations, and government. Large sets of consistent, annual data can be used in sea lice population studies, not only to determine abundance and intensity, but also to help map clustering patterns in predicting where sea lice will likely be most prevalent in a particular area by stage and time of year.
The program is currently synthesizing existing sea lice data sets into a useful format to communicate historical data trends, while also collecting and storing data from current industry-sponsored sea lice monitoring programs. This includes ongoing monitoring of sea lice on wild fish. By bringing these data together, new opportunities will arise to conduct further analyses, report on trends noted in various regions, and increase transparency as well as the potential for information sharing.
By integrating data sets from conservation groups, academia, First Nations, government, and salmon farming companies, greater analytical and modelling opportunities arise. This should ultimately benefit all contributing parties and the sustainability of the aquatic ecosystem along the BC coast.
Date: APR. 2016–MAR. 2017
Funded by: BC Salmon Farmers Association (BCSFA)
Project Lead: Crawford Revie (UPEI)
Project Team: Thitiwan Patanasatienkul (UPEI)
Contact: crevie@upei.ca
Website: http://www.upei.ca/avc/crawford-revie

The coastal waters of British Columbia. Photo: BCSFA
Susceptibility of Farmed and Wild Atlantic Salmon (Salmo salar) to Experimental Infestations with Sea Lice (Lepeophtheirus salmonis)
Sea lice (Lepeophtheirus salmonis) are common pests on farmed Atlantic Salmon and can have large economic consequences for the salmon industry. These consequences can include treatment costs, increased mortalities, and negative public perception. Sea lice originating from aquaculture farms may also negatively impact wild stocks of salmonids, although the extent of the impact is unclear. Salmonid species have been shown to have different susceptibilities to sea lice infection. Atlantic Salmon have been shown to exhibit substantial genetic variation (in addition to phenotypic variation) in resistance to sea lice.
This project will utilize controlled sea lice laboratory infestations to determine variations in sea lice susceptibility of three groups of fish: wild salmon from two origins (Garnish River, Conne River) and farmed salmon. Results from this project will: 1) aid the aquaculture industry in understanding and monitoring any potential wild-farmed interactions to ensure sustainable production of fish; 2) better inform on potential differences in sea lice susceptibility between farmed and wild salmon; and 3) provide information on stress levels for salmonids following sea lice infestations in all groups of fish.
Date: APR. 2015–JUN. 2017
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Cold Ocean Salmon Inc.
Project Lead: Dounia Hamoutene (DFO)
Project Team: Harry Murray, Lynn Lush, Kimberley Burt (DFO)
Collaborators: Julia Bungay (Cold Ocean Salmon Inc.)
Contact: Dounia.Hamoutene@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/16-1-G-02-eng.html
The Effects of Sea Lice in Modulating Salmonid Susceptibility to Viruses
The sea louse, Lepeophtheirus salmonis, is a naturally occurring parasite and a serious pest of farmed Atlantic Salmon in both Eastern and Western Canada. As sea lice are found on a number of wild host species in the marine environment and co-occur with endemic viruses, mixed infections of sea lice and viruses are likely. Despite the widespread occurrence of sea lice in both wild fisheries and aquaculture, there have been no controlled studies that have explicitly examined the effect of L. salmonis on disease caused by viral pathogens. Sea lice infected salmon may be more likely to develop severe infections with second pathogens, either because the sea louse serves as a vector, transmitting the secondary pathogens, or because the sea louse infection compromises the host immune response. The latter possibility is supported by the observation of reduced expression of genes associated with anti-viral responses and adaptive immunity in several salmon species during laboratory infections with L. salmonis.
This research project will focus on two viral pathogens, the Infectious Hematopoietic Necrosis Virus (IHNV) and the Infectious Salmon Anemia Virus (ISAV). IHNV infects wild and cultured salmonids throughout the Pacific Northwest of North America. ISAV infects and causes disease in farmed Atlantic Salmon in Eastern Canada. For both viral pathogens, there is a need to better understand if sea lice parasitism influences virus transmission and susceptibility of salmon to infection. This research project addresses this issue by integrating parallel investigations into IHNV and ISAV interactions with sea lice in Western and Eastern Canada, respectively. The goal of the study is to determine level of sea lice infestation at which intervention or pest management strategies may be needed to prevent further damage from viral infection. This research will provide scientific information for management decisions regarding sea lice infestation thresholds for use in salmon aquaculture.
Date: APR. 2014–MAR. 2017
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Lead: Simon Jones (DFO)
Project Team: Kyle Garver (DFO)
Collaborators: Mark Fast (UPEI–AVC)
Contact: Simon.Jones@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2014-P-12-eng.html
Defining the Risk of Sea Lice Infections Through the Development of an Understanding of the Early Life History Population Dynamics of Sea Lice Associated with Atlantic Salmon Aquaculture Sites in the Bay of Fundy
Sea lice continue to be a challenge for Atlantic Salmon farmers worldwide despite the development of various chemo-therapeutant treatments and advanced application measures (e.g., well boats). While some chemicals can be effective at controlling sea lice populations on the farm, they can sometimes affect other invertebrate groups and therefore need to be part of an Integrated Pest Management Plan (IPMP) that includes a variety of treatments to address various life stages of sea lice and are suited to varying environmental conditions. Nonchemical measures such as genetic selection for resistance or mechanical devices that can remove sea lice are currently being actively examined. All management techniques are ultimately premised on the ability to control the lifecycle of the sea lice from larvae to adult. However, there has been surprisingly little empirical field work done on the planktonic early life history stages of sea lice where the largest numbers of individual animals exist and the infection first spreads. This is due to the logistic difficulties of working in the field on commercial sites where production priorities take priority over scientific requirements, making it difficult to sample. Previous studies from this team conducted in the Bay of Fundy have revealed that the highest densities of sea lice larvae are consistently found around salmon farms, suggesting a specialised internal mechanism for retention. A better understanding of the early life history of sea lice in the Bay of Fundy will allow for insights on when to intervene in the lifecycle of the animal. Also, by assessing the degree to which aquaculture farms retain sea lice larvae and how they leave the site, we can begin to assess the risk to other farms and whether or not they could affect wild species in their spread.
Through this work, it may be possible to determine where the critical points are in the farming operation that may promote sea lice infestations and what may be done to reduce the infestations through protocol changes and engineering applications.
Date: APR. 2014–MAR. 2017
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Lead: Shawn Robinson (DFO)
Project Team: Emily Nelson, Steve Neil, Craig Smith (DFO)
Collaborators: Joel Halse (Kelly Cove Salmon Ltd.)
Contact: Shawn.Robinson@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2014-M-02-eng.html
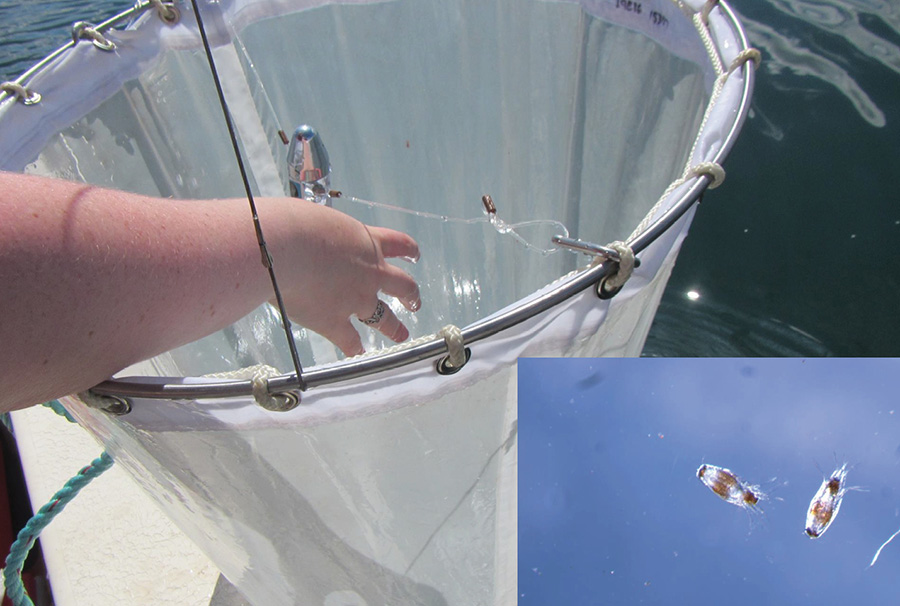
Plankton net with flow meter used to sample sea lice larvae (inset) in and around salmon farms in the Bay of Fundy. Photo: Shawn Robinson (DFO)
Development of Bacterial Biomarkers of Salmon Microbiota Mediated Resistance Against the Sea Louse Lepeophtheirus salmonis
Sea lice, Lepeophtheirus salmonis, parasitism of salmon represents an animal health issue for both wild and farmed salmon. Existing treatments are generally experiencing a loss of efficacy or variable results. Current research is exploring strategies such as vaccines, selective breeding, novel drugs, and non-chemical and biological control for the treatment, reduction, and removal of sea lice from farmed fish. This study represents a first step in developing strategies to reduce infections resulting from sea lice prevalence and sea lice landing by employing a probiotic approach.
Atlantic Salmon smolts were designated as non-exposed or exposed to a sea lice infection pressure of 40 copepodids per fish and monitored at regular time points as the sea lice developed from chalimus to large pre-adult. The mucosal and gut microbiome of salmon smolt was surveyed and population changes in response to L. salmonis infestation were examined. Study results suggest that sea lice infestation drives imbalances in salmon mucus microbiota, which could underlie some of the morbidity associated with sea louse infection. Principle component analysis and frequency distributions suggested that, over the course of the experiment, the sea lice exposed mucus microbiome became increasingly different over time, while water and biofilm remained similar. Although the control and treatment microbiome composition differed at the outset of the trial making strong assertions difficult, infected salmon microbiome changed more over time. This work demonstrates how a disrupted microbial community structure could allow for potential secondary infection to occur. Future research could examine ways to stabilize the community structure, reducing this risk.
This project provided a survey of the microbial ecology of farmed Atlantic Salmon. It also showed how the microbial community structure changed with respect to sea lice exposure and how the change increases with time post exposure as compared to non-infested salmon.
Date: APR. 2013–MAR. 2015
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Kelly Cove Salmon Ltd. (KCS); U Laval (NSERC–Engage)
Project Lead: Steven Leadbeater (DFO)
Project Team: Martin Llewellyn (Glasgow U); Keng Pee Ang (KCS); Nicolas Derome (U Laval)
Contact: Steven.Leadbeater@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/index-eng.html
Safety Assessment of an Oxygen Gas Infusion System Used During Various Hydrogen Peroxide (H2O2) Treatments on Atlantic Salmon (Salmo salar)
Numerous strategies have been developed to control sea lice infestations within the farmed Atlantic Salmon industry. Unfortunately, sea lice resistance to many chemotherapeutants has now been reported globally. Thus, the exploration of new therapeutants for sea lice is warranted and timely. Hydrogen peroxide (H2O2) has been used in combination with other products (e.g., SLICE, Salmosan) as part of a sea lice integrated pest management plan, but there are concerns regarding fish welfare when temperatures are high (>14°C) and fish are excessively stressed during treatment. One solution to minimize fish stress during treatment is to increase the dissolved oxygen saturation (via the GIS Gas Infusion System Transport Module) in the surrounding water, thereby creating a slight narcosis among the treated fish.
The Huntsman Marine Science Centre (HMSC) is currently evaluating the safety and efficacy of using a gas infusion system (to create 150% ± 10% O2 saturation while maintaining total gas pressure of 100%) when exposing Atlantic Salmon to H2O2 at varying treatment dosages (0-1500 ppm and exposure times 15-40 mins). Subsequent investigations will explore: 1) histological analysis of cellular and tissue-level sub-lethal effects; 2) additional dosages and exposure times; 3) the impact of using freshwater (e.g., bath treatment for amoebic gill disease, AGD); 4) the effect of various environmentally relevant temperatures; and 5) sea lice removal efficacy. Initial results are encouraging, with no ill-effects observed on commercially-sized salmon exposed to H2O2 dosages and exposure times that reflect industry practices.
This research is innovative and quite timely. New strategies to mitigate sea lice infestations, especially those that will have minimal to no impact on fish welfare, are needed globally.
Date: JAN. 2017–ONGOING
Funded by: GIS Gas Infusion Systems Inc.
Co-Funded by: New Brunswick Innovation Foundation (NBIF)
Project Lead: Duane Barker (HMSC)
Project Team: Anne McCarthy, Chris Bridger, Rebecca Eldridge, Esther Keddie, Ellen Fanning (HMSC)
Collaborators: Mike Beattie (GIS Gas Infusion Systems Inc.)
Contact: Duane.Barker@huntsmanmarine.ca
Website: http://www.huntsmanmarine.ca/
Developing a Non-Chemical Means to Effectively Remove All Forms of Sea Lice from Aquaculture Salmon Using Warm Water
The sea louse (Lepeophtheirus salmonis) remains a global challenge for salmon farming, with considerable resources expanded to manage this pest. Sea lice are becoming resistant to many of the traditional treatment chemicals which are also lethal to non-target organisms. Consequently, many non-chemical alternative treatments are being tested, including predators (cleaner-fish), traps (physiological or biological), and physical exclusion devices (nets, electrical fields).
A promising technique uses warm water showers to remove all attached stages of sea lice and prevent the detached sea lice from being returned to the ocean. This project aims to develop protocols for safe and effective showers to remove sea lice from Atlantic Salmon, and to understand why it works. Research results are expected to provide the required information for ongoing modification of the commercial sea lice warm water shower device, as well as inform sea lice management strategies.
Specifically, the following results were found:
- The use of a warm water shower will remove over 95% of all attached mobile stages of the sea lice at temperatures over 30°C.
- Mortality rates of fish going through the treatment are close to 0% for healthy fish, but slightly higher for fish that are in poor condition due to heavy infections.
- A small amount of surface mucus from the fish is removed during the process, but this is not significant.
- Lower salinities of the treatment water do not improve the removal efficiency.
- A technique was developed to quickly and easily quantitatively measure the mucus layer on a fish without harming the animal.
Developing a new, environmentally benign treatment option for industry will significantly increase the sustainability of the sector. The research is being done in close hands-on collaboration with the industry’s design engineers, healthcare professionals, and farm staff. The results are being applied to the next generation commercial prototype that is currently being designed.
This project supports the DFO objectives of environmental performance and optimal fish health.
Date: JUN. 2014–JUN. 2016
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Kelly Cove Salmon Ltd. (KCS)
Project Lead: Shawn Robinson (DFO)
Project Team: Steve Neil, Craig Smith (DFO); Joel Halse (KCS)
Collaborators: Keng Pee Ang (KCS)
Contact: Shawn.Robinson@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/M-14-02-001-eng.html

An adult egg-bearing female (gravid) of the salmon sea lice (Lepeophtheirus salmonis) removed using a warm water shower from an adult salmon. Photo: Shawn Robinson (DFO)
An Investigation of the Relationship Between Environmental Parameters, Oceanographic Zones of Influence, and the Prevalence of Parasitic Copepods on Three-Spine Stickleback in Bay D’Espoir Newfoundland with Specific Reference to Salmonid Aquaculture Sites
Sea lice infestation has increased with farmed salmon expansion in Newfoundland and Labrador’s Bay D’Espoir and Fortune Bay. The gill louse (Ergacilus labracis) was the most abundant sea lice species observed, but their impact on farmed salmonids has not yet been characterized. This study investigates the potential correlation between the distribution of sea lice on Three-Spine Stickleback and farmed salmonids in the region.
The results of this research will help provide information on the potential of wild non-salmonid fish species to act as sea lice reservoirs (with the potential to re-infect farmed fish), as well as a potential predictor of infestation levels in Bay Management Areas. The bays investigated in this study are defined by specific environmental conditions which can dictate how the sea lice community is partitioned. This study is one of the first to draw correlations between environmental conditions and sea lice ecology in the region.
This project supports the DFO objective of optimal fish health. Specifically, the following results were found:
- These bays can be divided in distinct zones based on environmental conditions.
- These zones are characterized by distinct populations of sea lice.
- Upper Bay D’Espoir is defined primarily by the presence of the parasite Ergasilus labracis and its relationship with Three-Spine Stickleback.
- Upper Hermitage Bay region is defined by the presence of the parasite Lepeophtheirus salmonis and its relationship with farmed Atlantic Salmon.
- The ecological needs of these different species likely act as partial biological barriers to their successful movement across zones.
Further monitoring is necessary to track the status of each parasite and continued diligence on the part of industry will be required to successfully manage the parasites prominent in each region.
Date: JUL. 2014–MAR. 2016
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Cold Ocean Salmon Inc.
Project Lead: Harry Murray (DFO)
Project Team: Andry Ratsimandresy, Alexandra Eaves, Sebastien Donnet, Dwight Drover, Sharon Kenny (DFO); Keng Pee Ang (Cold Ocean Salmon Inc.)
Collaborators: Cold Ocean Salmon Inc.
Contact: Harry.Murray@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/N-14-02-001-eng.html
Studies of Sea Lice Infection Levels on the Health of Juvenile Salmon in the Strait of Georgia and Adjacent Waters
To assess risk associated with sea lice and microbe transfer between farmed and wild salmon, we have undertaken a detailed study on the: 1) migratory pathways of wild salmon and the duration of their residency in the vicinity of fish farms; 2) the prevalence of pathogens and diseases within wild and farmed populations; and 3) the overall physiological well-being and health of wild populations as this impacts their susceptibility to infection. Using net-based surveys we have examined the duration of juvenile Fraser River Sockeye Salmon residency within the Strait of Georgia, as well as in the vicinity of salmon farms in the Discovery Islands. We have found that the majority of juvenile Fraser River Sockeye Salmon pass through the Discovery Islands over a two to three week period in early to mid-June. Caligus clemensi was the dominant species of sea lice present, but was in low abundance on juvenile salmon, Pacific Herring, and Three-spined Sticklebacks. There was no significant increase in the numbers of sea lice on juvenile salmon in the vicinity of salmon farms in the Discovery Islands. Infections with the Hematopoietic Necrosis Virus (IHNV) and the parasites Parvicapsula minibicornis and Myxobulus articus have been seen in samples collected in the lower Fraser River and throughout the Strait of Georgia. The prevalence of infection of these agents is highly variable among years and stock of origin. We have neither found Piscine Orthoreovirus (PRV) nor Renibacterium salmoninarum in any of the fish that we tested.
Date: APR. 2010–MAR. 2017
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR); DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP); Pacific Salmon Commission (PSC)
Project Leads: Stewart Johnson, Marc Trudel (DFO)
Project Team: Chrys Neville, Kyle Garver, Simon Jones (DFO)
Contact: Stewart.Johnson@dfo-mpo.gc.ca, Marc.Trudel@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2010-P-02-eng.html
Refining the Use of Warm Water Showers to Remove Sea Lice from Atlantic Salmon and Understanding the Fish Health Implications of the Technique
Considerable effort is expended (via chemo-therapeutants and animal husbandry practices) to manage parasitic infections by the sea louse, Lepeophtheirus salmonis, a globally acknowledged challenge for salmon farmers. The parasite is gaining resistance to treatments and new approaches are urgently needed. Recent research suggests that any technique must remove parasites from fish and also control the release of larvae and mobile parasitic stages within the farm which seem to contribute to the magnification of the infection cycles on the salmon leases for Southwest New Brunswick.
Over the last two years, a new technology was introduced to salmon farming in the Bay of Fundy in which salmon were exposed to a warm water shower. Through a series of laboratory and field trials, this technology was found to be very effective at removing mobile stages of sea lice (over 90%) and was able to retain virtually all of the removed sea lice, preventing them from being reintroduced into the water column near the salmon cages and re-infecting the fish. This project will refine and build on the results gathered from previous research through investigations on the effect of fish health on the efficiency of removal as well as the dynamics of the removal process itself.
Adding a new, environmentally benign treatment option for industry will significantly increase the sustainability of the sector and reduce the substantial investment in management controls by the regulatory sector. The research will be done in close hands-on collaboration with the industry’s design engineers, healthcare professionals, and farm staff. The results will be immediately applied to the next generation commercial prototype currently being designed.
This project supports the DFO objectives of environmental performance and optimal fish health.
Date: JUL. 2016–JUN. 2018
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Kelly Cove Salmon Ltd. (KCS)
Project Lead: Shawn Robinson (DFO)
Project Team: Steve Neil, Craig G. Smith (DFO); Joel Halse (KCS)
Collaborators: Keng Pee Ang (KCS); Duane Barker (HMSC)
Contact: Shawn.Robinson@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/16-1-M-01-eng.html

Testing the efficiency of the lab-based model of the warm water shower in the field with industry partners in the Bay of Fundy. Photo: Shawn Robinson (DFO)
- Date modified: