Canadian Aquaculture R&D Review 2009
Table of Contents
Sea Lice
BC Pacific Salmon Forum reports on interim research results
The BC Pacific Salmon Forum is an independent citizen body using science and stakeholder dialogue to advance sustainable governance of BC Pacific salmon. Three objectives were identified for a two year research program focused primarily in the Broughton Archipelago:
- To determine whether salmon farms in the Broughton impact sea lice loads on wild salmon and if so how;
- To determine whether the survival of individual wild fish is compromised due to increased lice loads; and
- To determine if any reduced survival of individual salmon has consequences on salmon populations, and if so, are there management techniques that can be put in place to mitigate any risk to wild salmon?
In 2008 the Forum provided funding to 15 collaborative research projects that involved more than 35 scientists. A wealth of new knowledge was gained in areas ranging from fish health and physiology, to oceanography, host-parasite interactions and the biology of sea lice. While the research focused predominantly on the Broughton Archipelago region, projects were also undertaken in other regions along the BC coast, some still ongoing. The findings from the 2008 research period built upon those from earlier seasons and will assist scientists in reaching a clearer understanding of ecosystem interactions in the Broughton and potentially beyond. The following provides a brief overview of the preliminary findings from the 2008 field season.
Modeling movements

Adding smolt to water column.
Aquatic organisms are in intimate contact with the ecosystems in which they reside and scientists are only just beginning to scratch the surface of the complex interactions between fish, parasites and pathogens, and the environments in which they coexist. The environment plays a defining factor in the outcomes of those interactions and a clearer understanding of the mechanisms of transport and exchange within the aquatic environment is vital to understanding the dynamics of salmon populations in our waters.
Oceanographic circulation and sea lice dispersion models have been developed for the Broughton Archipelago and have been utilized to simulate oceanographic conditions and lice concentrations for defined collection periodsFootnote 1. Ocean currents, salinities and temperatures in the model simulations were found to agree relatively well with data collected from the field leading to the expectation that these models may prove to be useful tools for investigating wild/farmed salmon interactions with sea lice and examining farm management strategies in the future.
Stock origins
One of the difficulties in understanding salmon dynamics is a lack of knowledge regarding the exact origins of a fish. Understanding the origins of each sample assists scientists in determining migration patterns and in identifying potential significant differences in infection patterns of different stocks. It is well known that different stocks of organisms do not share identical genetic makeup, some stocks may possess greater or poorer resistance to pathogens and this knowledge is useful in identifying stocks that may be at greater risk of impact from infection.
Micro-chemical analyses demonstrated that it is possible to discriminate the natal origins of pink and chum salmon sampled in the Broughton Archipelago based on otolith compositionFootnote 2. Juvenile salmon rearing in different stream systems are subjected to variations in water chemistry and these can be traced by micro-analysis. Salmon of known origin were correctly identified approximately 85% of the time suggesting that this research could provide a useful analytical tool in the ongoing efforts to better understand salmon migration pathways in the Broughton Archipelago.
Marine monitoring

Mike Sackville studies specimens under a microscope.
There has been a great deal of debate surrounding the dynamics of juvenile salmon and associated sea lice. While the answers still remain somewhat elusive, certain patterns are merging. Extensive sampling programs by several different research groups, over 5 or more years, have demonstrated that there are significant shifts in both salmon and lice populations, although these do not always appear to share a positive correlation. Total catches of juvenile wild pink and chum were high in 2003, but declined substantially for both species in 2004. Although total catches and catch-per-unit-effort (CPUE) remained relatively constant for both species from 2004 to 2008, the number of fish captured each year varied widely between sampling locations and between yearsFootnote 3. In 2008, a total of 622 sets were completed using beach seine and purse seine fishing gear with sampling occurring over 8-10 days each month, near the end of March, April, May and June. In 2008 a total of 22,995 juvenile pink salmon and 9,394 juvenile wild chum salmon were captured, very similar to the number captured in 2007.
It is generally considered that smaller fish are more susceptible to infestation by sea lice. In 2008, a large number of juvenile salmon were examined for sea lice. There were large variations in both incidence and severity of infection by L. salmonis on juvenile pink and chum salmon between years, and also between different locations within any particular yearFootnote 3 Footnote 4. The prevalence and abundance of L. salmonis on juvenile pink and chum salmon has continuously declined since 2004 although the explanations for this decline are not fully understood. The percent of lethally-infected pink salmon declined from approximately 1% in 2005 to zero in 2008. Lab studies have demonstrated that once fry reach approximately 0.7g they are highly resistant to the effects of liceFootnote 4.
A question that has yet to be clearly answered is ‘where do the lice come from?’. Farmed salmon are not infected when they enter the saltwater pens therefore, logically, the lice are of a natural source in the environment. However, we have yet to clearly identify that reservoir.
Sea lice management

This catch: mostly herring, few smolt.
It is accepted that there exists a relationship between sea lice on wild salmon and salmon farms, although that relationship is, as yet, incompletely defined. Regardless, prudence demands that a cautionary approach be taken to managing the interactions. Mathematical modeling has been used to connect data from farms with that from wild salmon sampling. SLICE® has been shown to reduce lice on farms and therefore transmission to wild juvenile salmon. Reduced sea lice transmission implies improved wild salmon survival but it remains unknown if SLICE® is sufficient to conserve and restore wild salmonFootnote 6. Studies in the Broughton indicate that infections begin in the winter. In the winter of 2008, salmon farms at the Knight Inlet/Tribune Channel region were treated with SLICE® and thus were virtually free of sea lice. In the winter of 2007/2008 sticklebacks around these farms were heavily (~70%) infected with chalimus stages of C. clemensi, an infection that occurred throughout the winter and continued after SLICE® was administered. The prevalence and intensity of the infection before and after treatment indicated that C. clemensi was being continually produced from a source other than the salmon farmsFootnote 5.
An important component of management is an understanding of the range and density of lice in the environment. In an attempt to quantify the abundance and map the distribution of larval lice in the region, bi-weekly plankton tows were conducted throughout the Broughton in 2007-2008Footnote 7. The abundances of naupliar and copepodid stages of both Lepeophtheirus salmonis and Caligus clemensi were low. L. salmonis were found to be most abundant near recently active fish farms while C. clemensi showed little or no spatial association with fish farms, but may have been associated with herring aggregations.
Host-parasite interactions
That sea lice migrate towards the surface at night and move deeper during the daylight hours has long been accepted. However, recent research suggests that this may be less clear than previously thought. When L. salmonis nauplii and copepodids were held in a 10 m column suspended in the ocean there did not appear to be a preference for any particular depth and there was no apparent effect of daytime period on their vertical distributionFootnote 8. In the absence of lice, juvenile pink salmon distribution changed slightly from day to night; fish less than 3 weeks post-saltwater entry (up to ~0.5 g) were typically found to occupy the top 3 m of the water column. When lice and fish were held together in the column, salmon dispersed more during the day, but their pattern of distribution did not change at night. No difference in the proportion of infected vs. uninfected fish was observed relative to the vertical distribution of the fish, suggesting that infective pressure is independent of daylight and depth. There have been suggestions that juvenile salmon prey on the eggs of lice and/or the lice themselves. It is unknown whether the copepodids were following the fish as potential hosts or the fish were following the copepodids as a potential food source, only that their vertical distributions were correlated within the column when they were free to interactFootnote 8.
Physiological implications of infection
While much of the focus in recent years has been on population effects of lice loads, there is very little available data on the effects of infection on individual fish. Experiments in 2008 involved over 10,000 juvenile pink salmon, some as small as 0.2 g, and examined in a field setting, the effects of L. salmonis on ionic balance and swimming performance of the fish at sea lice densities of 1 to 3 lice per fish. Sub-lethal effects of 1 louse per fish on ionic balance and swimming performance were dependent on the size of the fish but significant effects occurred only on fish with a body mass of <0.5 g. A higher density of lice (2 or 3 lice per fish) was required to trigger sub-lethal effects on ionic balance and swimming performance in larger fish (0.5 – 3.7g). In all cases, sub-lethal effects were only detected when the life stage of the louse was at least chalimus 3-4. Compared with the sub-lethal changes observed for <0.5 g fish with 1 louse per fish, increasing lice density up to 3 lice per fish did not have an additive sub-lethal effect for fish of any sizeFootnote 9. Interestingly, naturally-infected fish appeared to be less sensitive to lice loads than did those fish that were artificially infected under lab conditions. Regardless, in all physiological studies mortality was minimal (<2%) among fish infected with sea lice during tests that lasted up to 28 days. Additionally, there is evidence from more than one study that juvenile salmon may possess effective mechanisms for shedding liceFootnote 5 Footnote 9.
Fish health
Fish health is a complicated field and involves a great deal more than a simple host-parasite interaction. Fitness is impacted by early rearing, natural feed supplies, genetics, environmental conditions, etc. Many factors lead to the state we call disease, and a pathogen is merely one factor in a complicated equation. Merely finding a parasite or pathogen on or in any organism is not to be confused with a state of disease. If a fish is in a healthy state it will be quite capable of combating any number of challenges. When that same animal is compromised by some factor, biotic or abiotic, the scenario can play out quite differently. For this reason it is important that we gain knowledge regarding the overall health of a population of fish.
Juvenile pink salmon out-migrating through the Broughton Archipelago in 2008 were evaluated for physiologic condition, sea lice, other parasites, bacteria, viruses, and microscopic lesions. Histopathology found microscopic lesions that are considered to have developed in the saltwater environment, yet were not attributable to sea liceFootnote 10. This suggests that there are other, as yet unidentified, factors that are impacting juvenile salmon in the Broughton. In nature, parasites are a normal and natural occurrence but the question remains, are salmon unhealthy because they are infested with lice, or are they infested with lice because they are unhealthy?
Salmon population dynamics
It is important to highlight the fact that salmon populations have shown significant declines all along the BC coastline. The population depressions are not limited to the Broughton. However, the research funded by the Forum focused on this region and found that pink salmon production out of the Glendale Channel was approximately half of that of 2007 and this is likely attributed to low adult escapement in 2007 relative to higher numbers observed in 2006Footnote 11.
Installation of a resistivity counter on the Glendale spawning channel will allow accurate egg–to-fry survival numbers to be acquired over the coming years to assess survival trends over time. Preliminary estimates of escapement derived via over-flight assessments indicate replacement of brood in some systems and much reduced returns relative to brood in other systems. The overall trend in escapement indicates a significant decrease in overall numbers between 2006 and 2008, with the Glendale at 91% fewer numbers of returning adults than in 2006. The trends are similar to those observed in other systems outside of the Mainland Inlets (i.e., Central and North Coast) and do not appear to be a localized event as was encountered in 2002 and 2003Footnote 11.
Salmon/lice dynamics in regions outside the Broughton Archipelago

Water column raised for inspection.
While the overall focus of the Forum-funded research was the Broughton Archipelago, there were research projects underway in other regions that were fully or partially funded by the BC Pacific Salmon Forum. Juvenile salmon in the Bella Bella region, an area that lacks salmon farms, were found to host low levels of sea lice (3.5%), which are considered to be natural background levels for the region. In regions where there are fish farms, significantly more juvenile salmon were found to host sea lice in areas near to farms compared to areas farther from farmsFootnote 12. Elevated levels of lice nearer to farms included significantly greater proportions of L. salmonis, a salmon-specific species, than C. clemensi, which is more of a generalist (found on numerous fish species).
The Kitasoo Fisheries Program undertook a monitoring program and collected juvenile salmon in areas around and away from salmon farms between 2004 and 2008 and assessed them for sea lice. Both C. clemensi and L. salmonis were observed on fish in all areas sampled. In 2008, lice levels were the lowest of all years data were collected. Prevalence and intensity of L salmonis was low in all areas sampled and in all years examined for both chum and pink salmon. L. salmonis abundance levels were higher on pink salmon caught around farms in 2005 and 2006 but not in 2007 or 2008 when data were compared to the reference areaFootnote 13.
The Gulf Islands area in the Strait of Georgia is a major rearing area for all species of juvenile Pacific salmon. As part of another study in 2008, researchers were able to measure sea lice levels on these juvenile salmon. The levels of infection were approximately 70% and were mostly C. clemensi. There were no salmon farms in the area, indicating that large, natural infections of sea lice can occurFootnote 5.
Conclusions
The purpose of the Broughton research strategy was to attempt to clarify some of the interactions between farmed and wild fish in the marine ecosystem with a primary focus on wild pink and chum salmon populations in all facets of their life cycle. The research considered what factors in the ecosystem may impact these wild fish populations, the incremental risk, if any, associated with salmon farming, and how any potential risks could be mitigated. While many questions remain unanswered and new questions have arisen as a result of a rigorous collection of scientific investigations, the research findings expand our existing knowledge and will continue to provide a foundation for future studies that will shed more light on the inter-relationships among all factors.
Note: This article is based on individual research summaries provided to the BC Pacific Salmon Forum and the information has not been subjected to a formal peer review process. The full summaries of the research projects highlighted within the above may be found at the BC Pacific Salmon Forum’s website at www.pacificsalmonforum.ca
Duration: ’06 – ‘09
Funded by: BC Pacific Salmon Forum
For information contact: bcpsf@pacificsalmonforum.ca
Website: www.pacificsalmonforum.ca
Measuring the overall health of out-migrating juvenile pink salmon
There is considerable discrepancy in the interpretation of findings between lab-based and field sea lice studies conducted in British Columbia. Moreover, little information is available on the effects of other parasites and lesions on wild pink salmon at the individual and population levels.
In order to resolve this situation, researchers have been investigating the overall health of juvenile pink salmon during their out-migration in the Broughton Archipelago in 2007 and 2008. The fish were evaluated for physiologic condition, sea lice, other parasites, bacteria, viruses, and microscopic lesions.
This investigation yielded a number of interesting results. Juvenile pink salmon collected directly from the Glendale River in March 2008 had no sea lice or microscopic lesions suggesting that all lesions in fish sampled from salt water developed after saltwater entry. In 2008, the highest prevalence of motile sea lice occurred in June. Condition factor was not significantly associated with sea lice. Maximum prevalence of fish with acutely damaged liver cells (hepatocellular hydropic degeneration) was greater in 2007 (32%) than in 2008 (12%). Sea lice were negatively associated with liver glycogen stores in 2008. Parasites belonging to several taxonomic groups infested the Juvenile pink salmon, in many cases at higher prevalence levels than sea lice.
Duration: Mar ‘07 – Nov ’08
Funded by: BC Pacific Salmon Forum
Project team: Sonja Saksida (BC CAHS), Gary Marty (BCMAL), Simon Jones (DFO), Sophie St-Hilaire (Idaho State U)
For information contact: Sonja Saksida ( sonja.saksida@cahs-bc.ca)
Monitoring reveals decline in sea lice prevalence and abundance on wild salmon juveniles
In the Broughton Archipelago the prevalence and abundance of L. salmonis on wild juvenile pink and chum salmon has continuously declined since 2004. The reasons for this decline are not yet fully understood. DFO has conducted surveillance for sea lice since 2003.
In a controlled laboratory experiment, approximately 35% pink salmon weighing less than 0.3 gram died following exposure to L. salmonis copepodids. But by the time these juvenile pink salmon grow to about 0.7 grams, they are highly resistant to the lethal effects of L. salmonis. From this research the threshold of lethal infection was calculated to be of 7.5 lice per gram for pink salmon weighing less than 0.7 grams.
The monitoring in the Broughton Archipelago has shown that in all years, virtually all wild pink salmon (> 98.9%) sampled in late March and early April had a mass of less than 0.7 grams. But by June, in all the years surveyed, less than 1% of wild pink salmon juveniles are still under 0.7 grams. The monitoring also revealed that the percentage of wild pink salmon weighing less than 0.7 grams with infections greater than 7.5 lice per gram was 4.5% in 2005, 0.8% in 2006, 0.4% in 2007 and zero in 2008.
Duration: Mar ‘03 – Nov ’08
Funded by: BC Pacific Salmon Forum, DFO-ACRDP. Co-Funded by: Marine Harvest Canada, DFO.
Project team: Simon Jones (DFO), Brent Hargreaves (DFO)
For information contact: Simon Jones ( simon.jones@dfo-mpo.gc.ca)
Multi-disciplinary team investigates genomics in sea lice and salmon
A new multidisciplinary research team in British Columbia initiated a project called Genomics in Lice and Salmon (GiLS). This 3-year project involving collaboration between three universities, industry partners and provincial and federal governments is aimed at achieving four key objectives.
First, researchers expect to elucidate Pacific and Atlantic salmon genetic responses following laboratory exposures to the sea lice Lepeophtheirus salmonisusing salmon microarray technology. This knowledge should enable them to predict defense responses in both susceptible and refractory host species.
Second, the team is working to uncover the parasite genetic responses in order to develop effective parasite treatment targets. They plan to do this by obtaining a comprehensive list of the genes of L. salmonis and Caligus spp. and building a gene chip to examine gene expression patterns following exposure to environmental or biological effects.
Third, the team intends to confirm and define the newly identified Pacific population of L. salmonis. They are using existing and novel microsatellite markers and single nucleotide polymorphisms (SNPs) to examine the amount of genetic variation and its distribution in Pacific populations of sea lice.
In the fourth component of the project, researchers will analyze the potential for effective use of science in understanding, and ultimately moving towards the resolution of the sea lice/salmon controversy.
The full journal article of this project’s results can be viewed at: http://springerlink.com/content/u63k14u857p40387/
Duration: Nov ‘08 – Oct ’11
Funded by: Genome BC. Co-Funded by: U Vic, DFO, SFU, VIU, Marine Harvest Canada, Mainstream Canada, Grieg Seafoods, Microtech Research and Development Ltd., BCMAL
Project team: Ben Koop (U Vic), Simon Jones (DFO), Willie Davidson (SFU), Grant Murray (VIU)
For information contact: Ben Koop ( bkoop@uvic.ca)
Can SLICE® have an impact on non-target organisms?
Sea lice infestations at marine cage finfish farms in British Columbia are often treated by the anti-parasitic chemotherapeutant SLICE®. Concerns have been raised by various stakeholder groups regarding the potential effect of the active compound in this treatment, Emamectin benzoate (EB), on non-target organisms.
To address this concern, researchers are investigating the fate of EB and its desmethyl metabolite following the application of SLICE® at selected finfish farm sites in the surrounding environment. They are measuring concentrations in localized sediments and the water column in order to provide an assessment of the potential effect on ecosystem functioning. The team is also looking at the uptake of EB and its desmethyl metabolite by the Pacific Spot Prawn,Pandalus platyceros, under both laboratory and field conditions.
This dual approach is providing a standardized framework for toxicity assessment and evaluation of ‘real world’ environmental conditions against which the toxicity measures can be compared. The outcomes of the laboratory study are providing a framework within which field derived measurements can be assessed. In addition, the measured environmental EB concentrations are being used to test, calibrate and implement the DEPOMOD model so it can be used to predict the behavior of EB in relevant aquatic ecosystems. These findings are proving to be useful in developing policy towards the regulation of SLICE®.
Duration: Sep ‘08 – Aug ’09
Funded by: BC Pacific Salmon Forum. Co-Funded by: DFO-PARR, BCMoE
Project team: Michael G. Ikonomou (DFO), Jon Chamberlain (DFO), Graham van Aggelen (EC), Caren Helbing (U Vic), Eric McGreer (BCMoE), Chris Sporer (Pacific Prawn Fishers Association)
For information contact: Michael G. Ikonomou ( Michael.Ikonomou@dfo-mpo.gc.ca)
Researchers test light traps to monitor and control planktonic-stage sea lice
Investigators at Simon Fraser University and DFO are looking at novel ways to monitor and control sea lice using light traps. Sea lice are parasitic copepods that attach to and feed on fin fish. Aquaculture operations are striving to minimize sea lice infection within fish farms and prevent transfer between cultured and wild fish. Infection of both wild and cultured fish occurs when they encounter larval sea lice during their brief free-living planktonic stage of the life cycle.
Present control strategies minimize production of planktonic lice by limiting the density of their parasitic parent population. Presently this is done by adding the pesticide SLICE® to the fish feed when parasites on the penned fish exceed a triggering threshold abundance. However, the treatment is expensive, and cannot be administered close to harvest date.
A supplementary control could involve elimination of the planktonic stage lice. Both adult and planktonic sea-lice are positively phototactic: they swim toward a point source of light. This behavior can be exploited to physically trap the lice. Light traps are being deployed within and near fish pens in the Broughton Archipelago. By comparing trap capture rates with ambient densities of planktonic lice, sampled by pump, an estimate of the mortality or removal rate per trap can be made.
The team is also making a limited number of net tows in a region where planktonic lice were relatively abundant in 2007 and 2008, to learn if enhanced SLICE® treatment and fallowing in 2009 is having the desired effect.
Duration: Sep ‘08 – Mar ‘09
Funded by: DFO-PARR
Project team: David Mackas (DFO), Moira Galbraith (DFO), Brian Riddell (DFO), Iñigo Novales Flamarique (SFU), Christina Gulbranson (SFU)
For information contact: David Mackas ( Dave.Mackas@dfo-mpo.gc.ca)
Modeling supports coordinated area management production in the Broughton
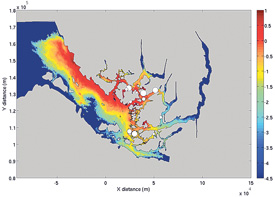
Concentration of infective sea lice (Log10[copepods/m3 ] ) arising from farm sources. White circles show farm locations and size of the circles represents strength of the source.
The Broughton aquaculture industry has recently proposed a Coordinated Area Management Production (CAMP) plan in which a combination of SLICE® treatments and fallowing are used to minimize potential lice infections on wild juvenile salmon migrating seaward past fish farms.
As part of a BC Pacific Salmon Forum project, researchers Dario Stucchi and Mike Foreman have developed two computer models for the Broughton Archipelago. One is a three-dimensional numerical circulation model that, with appropriate forcing for specific time periods, is capable of simulating velocity, salinity and temperature fields throughout the region. The second is a model of sea lice dispersal, development, and behaviour. This model applies the 3D circulation model of currents to predict dispersal of the planktonic larvae and model the temperature and salinity fields which control sea lice development and mortality.
As a guide to the future implementation of CAMP and in consultation with industry, the two models are being used to investigate how the 2008 infective pressures would have changed under different treatment/fallowing scenarios.
Duration: Sep ‘08 – Mar ’09
Funded by: DFO PARR Co-Funded by: BC Pacific Salmon Forum
Project team: Dario Stucchi (DFO), Mike Foreman (DFO), Ming Guo (DFO), Piotr Czajko (U Vic), Gabe Wiebe (U Vic), Brent Hargreaves (DFO), Simon Jones (DFO), Marine Harvest Canada, Mainstream Canada, Grieg Seafood BC Ltd.
For information contact: Dario Stucchi ( Dario.Stucchi@dfo-mpo.gc.ca)
Might sea lice be a vector for other pathogens?

Sea lice bisected for bacterial sampling / Bacteria colonies isolated from lice (Photo: D Barker)
The potential role of sea lice in a vector-pathogen association affecting farmed salmon has not been explored. Using standard OIE bacteriological screening protocols, a Vancouver Island University researcher and his students sampled the external carapace and internal stomach contents of motile sea lice (Lepeophtheirus salmonis, Caligus clemensi) collected from farmed Atlantic salmon from May 2007 to April 2008 in British Columbia.
The results of this pilot study include the first isolation of three potentially pathogenic bacteria (Tenacibaculum maritimum, Pseudomonas fluorescens andVibrio spp.) from sea lice and their corresponding healthy salmon hosts. Spatiotemporal variation among prevalence of bacteria was evident from external (58-100%) and internal (12.5-100%) sea lice samples. There was also an increase in prevalence of bacteria cultured from lice collected from higher seawater temperatures and age of lice (90-100% among adult lice compared to < 40% among pre-adult).
These preliminary results have lead to an NSERC proposal to conduct a comprehensive, long-term study in which this team intends to explore, within an ecological context, the role of sea lice as vectors of pathogens to salmon. Where (geographically) and when (seasonally), could sea lice carry important pathogens of salmon? A further question to explore is can sea lice be used as indicators of pathogens in an environment?
Duration: May ‘07 – May ’08
Funded by: VIU. Co-Funded by: Marine Harvest Canada, BCMAL.
Project team: Duane Barker (VIU), Laura Braden (VIU), Mark Sheppard (BCMAL), Maria Coombs (BCMAL), Brad Boyce (Marine Harvest Canada)
For information contact: Duane Barker ( Duane.Barker@viu.ca)
Juvenile pink salmon must reach critical weight to withstand sea lice
Controlled laboratory exposures to L. salmonis were used to assess the effect of host size and parasite dose on the susceptibility and survival of wild- and hatchery-spawned pink salmon. The researchers conducted three trials in which juvenile pink salmon had starting weights of approximately 0.3, 0.7, and 2.4 grams. In each trial, the fish were exposed to 0, 25 (only for 0.3 g), 50, or 100 copepodids per fish.

D Barker & L Braden in bacteriology lab (Photo: D Barker)
The results revealed that regardless of the pink salmon stock, approximately 37% of the 0.3 g fish died following exposures to 50 or 100 copepodids. The mean days to death was 16.1 and more than 80% of the lice on the dead fish were in the chalimus stages. Mortality was significantly lower when the larger fish were exposed to the sea lice. The researchers surmise that the skin of the 0.3 g pink salmon is histologically poorly developed and lacks scales compared with skin of 0.7 g and 2.4 g pink salmon.
The results indicate that an elevated risk associated with L. salmonis infection may occur among migrating post-emergent pink salmon during a relatively brief period before the fish reach 0.7g.
The full journal article of this project’s results can be viewed at: http://www3.interscience.wiley.com/journal/120751387/issue
Duration: Apr ‘07 – Jun ’08
Funded by: DFO-ACRDP. Co-Funded by: Marine Harvest Canada
Project team: Simon Jones (DFO), Eliah Kim (DFO), William Bennett (DFO)
For information contact: Simon Jones ( Simon.Jones@dfo-mpo.gc.ca)
Will Scottish sentinel cage technology work in the Broughton Archipelago?
Health management of cultured salmon in British Columbia includes scheduled monitoring of parasitic sea lice, including Lepeophtheirus salmonis. Farmed stock is treated if sea lice infestations exceed the threshold defined in the Provincial Sea Lice Action Plan. Although infestations of farmed Atlantic salmon persist, associated disease has been virtually absent in BC and the parasite is considered a nuisance.
The extent to which local environmental (e.g., salinity, temperature, currents) and biological (e.g., abundance, distribution and diversity of wild hosts) processes not associated with salmon farming regulate levels of sea lice risk within the Broughton Archipelago is poorly understood. There is very little information on the abundance and distribution of wild salmonid hosts in the Broughton Archipelago particularly in the winter months. Consequently, farmed Atlantic salmon continue to be implicated as the most abundant hosts.
A method for quantifying the abundance of planktonic sea lice larvae (copepodids) during the winter months is needed, particularly during the weeks and months prior to the out-migration of juvenile pink and chum salmon. The objective of the proposed research in this pilot study is to determine the feasibility of using proven Scottish sentinel cage technology to better characterize the distribution of infective sea lice stages in the Broughton Archipelago.
Duration: Dec ‘07 – Sep ’09
Funded by: DFO-ACRDP. Co-Funded by: Marine Harvest Canada
Project team: Simon Jones (DFO), Sonja Saksida (BC CAHS)
For information contact: Simon Jones ( Simon.Jones@dfo-mpo.gc.ca)
- Date modified: