Canadian Aquaculture R&D Review 2007
Finfish - Marine
Tougher cage systems the goal of East Coast research
Most people who have not seen or experienced it themselves really have little idea of just how brutal ocean conditions can be for fish farming on the East Coast of Canada, according to the general manager of a net pen construction, design and repair company in the region.
So GMG Fish Services Ltd., a subsidiary of Cooke Aquaculture, has an ongoing program to work with scientists and researchers to continually improve net pen cage-system designs for the salmon-farming industry. The program is based out of the company's net-pen design and manufacturing facilities in St. George, New Brunswick.
GMG Fish Services GM Alan Cook has experience with fish-farming on both the Canadian West Coast and East Coast, and he says there is truly little comparison between what East Coast farmers have to contend with, and the much more sheltered conditions on the West Coast.
In particular, said Cook, many of the East Coast farm sites are exposed directly to the open Atlantic Ocean, so the "fetch" or distance over which wind and waves can build is considerable.
To counter that, GMG has been working with the Institute of Ocean Technology's Dr. Bruce Colbourne in Newfoundland to come up with ways for net pen mooring attachments to absorb more of the shock from waves and currents.
The research, which is supported by the Industrial Research Assistance Program of the National Research Council, is aimed specifically at coping with extreme conditions in a deep-sea farming setting.
Tests are continuing on aspects such as different mooring-grid depths, the effect of weight rings, and a modified bird-net stand to handle the heightened problems East Coast farmers have not just with cormorants but also with seals.
Cook said that one of the big problems for East Coast fish farmers is that waves that measure three to four meters from crest to trough are not an uncommon feature on the coast.
"Those are significant waves, and you can get them 10-metres high in the open ocean out here," he said, adding that currents of two knots and more are also a comparatively frequent occurrence. Heavy ice loads in winter also have to be taken into account.
So GMG has been working with the institute to improve net-cage systems so they can handle that kind of marine assault, using a scale model of cage systems in the institute's 100-by-30-metre wave-simulation tank.
"We're trying to adjust improvements to the mooring systems, to reduce the shock loading on the moorings from high waves and currents and find ways to control the motion of the bottom panel of the net," he said.
Cook, who recently made a presentation on pen design to a conference on off-shore fish-farming in New Hampshire, said that one way East Coast fish farmers try to control or reduce the motion of the bottom of the net is through using weight rings, but he thinks that few people have much experience with them.
He also noted that there is a considerable difference between the submerged, research sized open-ocean cage systems which have drawn so much attention off the United States, and the industrial-scale cages GMG makes - mostly, to this point, for internal use within Cooke Aquaculture.
"The design and the materials (for the submerged systems) won't allow them to be scaled up to the high-volume output size the industry wants and needs," he said. He said to scale them up to that - from roughly 6,000 to about 10,000 cubic metres - would hike the cost of the materials to about $30-35 a cubic metre, which would be prohibitive.
Submitted by the Institute of Ocean Technology. For more information contact Bruce Colbourne (Email: D.Colbourne@nrc-cnrc.gc.ca)
Exposing Atlantic cod to Nodavirus
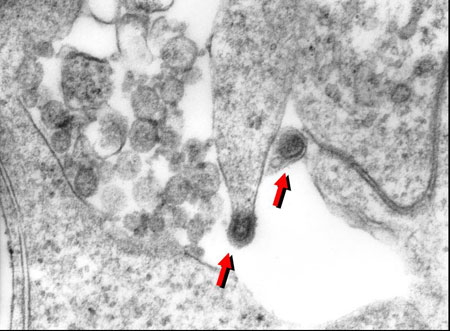
Betanodavirus particles isolated from Atlantic cod, in cytoplasm of susceptible (E-11) cells.
Viruses of the family nodaviridae have been reported to cause the debilitating disease, termed either viral nervous necrosis (VNN) or viral encephalopathy and retinopathy (VER), in a wide variety of marine fish hosts throughout the world. The presence of betanodavirus in wild Atlantic Canadian cod adults was reported by Cusack and colleagues in 2002, with clinical disease recently confirmed in hatchery-reared cod juveniles from eastern Canada and USA. These clinical outbreaks have resulted in high levels of morbidity and mortality, supporting the hypothesis that betanodavirus poses a serious threat to the successful commercialization of Atlantic cod aquaculture in Canada.
The partial sequencing of a recently isolated NNV from striped bass in New Brunswick revealed variations and a distantly related virus in comparison to local isolates that were involved in previous outbreaks.
In this project, we injected juvenile cod with either a NNV isolated from haddock (known to cause disease in cod), or the striped bass isolate. Fish were sampled every month for one year and their tissues (spleen, kidney, eye, brain, blood) were tested for the presence of nodavirus by virology and RT-PCR. A new RT-PCR assay was designed to better match NNV sequences. Preliminary results show that the striped bass isolate did not kill cod, but fish remained positive for NNV more than a year after exposure.
Research team: Nellie Gagné, Anne-Margaret MacKinnon, Paul Harmon For information contact Nellie Gagné (Email: gagnena@dfo-mpo.gc.ca). Submitted by DFO(ACRDP).
Apr. '05-Mar. '07
Improving diagnosis of Betanodavirus in Atlantic Cod
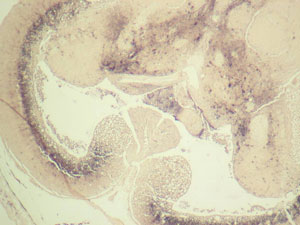
Betanodavirus in the brain of a cod.
To support the developing Atlantic cod aquaculture industry in Canada we are developing rapid, cost-effective pathogen screening methods with known performance characteristics for pathogens of Atlantic cod. Rigorous assessments of how diagnostic tests perform in clinical samples are required to take appropriate decisions regarding pathogen detection and control. To this end an initial collaborative research program examining the pathobiology of Atlantic cod nodavirus was recently undertaken by researchers at DFO (Moncton), NRC (IMB Halifax), Aquatic Diagnostic Services (AVC - UPEI), the University of Waterloo and industrial partners as part of an AquaNet project. This work has lead to the development of new or modification of existing diagnostic methods for use on strains of nodavirus that have been found in Atlantic Canadian waters.
Our current research activities, funded through the DFO industry sponsored ACRDP, are focused on optimizing the application of these methods for the screening of different life history stages and tissues of Atlantic cod for nodavirus. Techniques developed during this program will be available for use by federal and provincial agencies and commercial diagnostic laboratories in Canada. Improved disease surveillance will reduce the potential for losses due to nodavirus disease and ultimately improve the competitiveness of Canadian Atlantic cod farming.
Research team: Nellie Gagne (DFO), David Groman (AVC), Tokinori Iwamoto (AVC), Carmencita Yason (AVC), Stewart Johnson (NRC-IMB), Daryl Whelan (DFO), Larry Hammell (CAHS - AVC), Jane Symonds (CGP). For information contact Nellie Gagne (Email: gagnena@dfo-mpo.gc.ca). Submitted by DFO (ACRDP).
Sept. '06-Mar. '08
Selective breeding for elite Atlantic cod
Aquaculture often relies on wild populations for broodstock, which is the situation with Atlantic cod farmed in Canada. Broodstock selection is essential in order to produce cod that perform well in captivity.
Until now, researchers in Atlantic Canada have used communal spawning to establish cod production runs. This approach has been used for haddock as well, and recent analyses of fish demonstrate a low genetic diversity of fish produced (primarily due to the dominance of individual males in group spawning situations). This project will implement established paired mating breeding protocols used in Norway to set-up cod families in Canada. Families will be reared separately until the 10-20 g phase, pit tagged, and stocked in sea cages. They will be grown to market size, and based on family performance elite broodstock will be selected for future use.
This study has six objectives:
- Development of broodstock reproduction and spawning protocols.
- Establish families and evaluation of early rearing performance.
- Examine effect of egg batch quality on family performance.
- Evaluation of sea cage performance and trait analysis.
- Conduct preliminary heritability estimates.
- Selection of elite broodstock.
Research team: Ed Trippel, Joe Brown, Steve Neil, Paul Harmon, Jake Elliot, Mike Szemerda, Frank Powell, Kjersti Fjalestad, Lynn Lush, Ian McMillan, George Nardi, Richard Rideout, Sharen Bowman, Jane Symonds. For information contact Edward Trippel (Email: TrippelE@mar.dfo-mpo.gc.ca). Submitted by DFO (ACDRP).
Jun. '05-Mar. '08
Developing the technology for culturing juvenile copper rockfish

The overall goal of this new project is to begin to develop the necessary technology to cost effectively culture copper rockfish (Sebastes caurinus) throughout their juvenile life history. Upon successful completion of the experimental work, considering both biological and economical perspectives, it is planned to locate the rockfish and to apply the acquired information at a land-based culture facility that will be established by Ko-Un Fish Company Ltd., on the West coast of BC.Here, the copper rockfish will be grown to market size.
In the present study, we plan specifically to:
- Develop a premium quality cost effective formulated diet of optimal buoyancy for culturing juvenile copper rockfish. Known information about the nutrient and energy needs and acceptable dietary components of Korean rockfish will be used as a reference guide. Low capital cost pelleting equipment together with vacuum microwave drying technology will be employed.
- Establish the dietary protein and energy requirements of juvenile copper rockfish.
- Establish baseline health criteria for juvenile copper rockfish and then assess the health of this species during culture.
Research team: D.A. Higgs, T. Durance, S.K. Balfry, M. Rowshandeli, C-H. Huang, and P. Konken. For information contact D. Higgs (Email: higgsd@dfo-mpo.gc.ca). Submitted by DFO (ACRDP).
Jul. '06-Apr. '07
Using photoperiod to manipulate growth and maturation of Atlantic cod
Impressive results and savings from a study assessing the effects of photoperiod on growth and maturation of Atlantic salmon in the Bay of Fundy , leads to the question of whether photoperiod manipulation on sea cages can be successfully applied to other finfish in Atlantic Canada.
In preliminary trials, early maturation in pre-market Atlantic cod affects ~100% of fish in sea cages. Thus, the maturity problem for cod is worse than for salmon. Repeated maturation of individual fish may occur before marketing as both males and females may achieve sexual maturity before attaining market size. Coincident with maturation and shedding of gametes in sea cages is a seasonal 25% weight loss in females and 12% in males. Appetite loss also occurs during spawning which leads to slower growth. During spawning and afterwards, fillets become 'jellied' (high water content) and are reduced in market value. This study will evaluate a method to decrease early maturation.
Other light problems also exist. Groundfish such as cod inhabit waters of 100 m depth (common range 30-300 m) where light intensity is very low. Species specific differences exist in the production of "sunscreen", yet no investigation has been undertaken to examine levels of sunscreen in a gadoid species such as cod. The molecules necessary for synthesis of "sunscreen" are obtained directly from the diet, and thus fish fed on artificial diets may be more susceptible to UV radiation than wild fish. Use of shade cloth on sea pens may be necessary to inhibit stress due to UV radiation for groundfish such as cod.
The primary objectives of this project are:
- To adapt technology proven for species such as Atlantic salmon to Atlantic cod.
- To examine the degree of suppression of maturation in both sexes in pre-market and market fish.
- To examine the potential for somatic growth enhancement in light treatments.
- To examine "sunscreen" production and possible use of shade cloth in connection with 24 h lights.
Research team: Ed Trippel, Steve Neil, Chris Duffy, Paul Harmon, Andrew Davie, Jake Elliot, Mike Szemerda, Frederique Kandel, Frank Powell. For information contact Ed Trippel (Email: TrippelE@mar.dfo-mpo.gc.ca). Submitted by DFO (ACRDP).
Dec. '04-Mar '07
Evaluating grow-out performance of Atlantic halibut


Tagging and weighing halibut
Atlantic halibut, a white-fleshed fish with high market value and demand, is an excellent species to complement and diversify the Atlantic salmon aquaculture industry. Atlantic Canada's aquaculture industry is now poised to take advantage of investments made in developing local expertise and infrastructure in hatchery production of coldwater marine fish. However, given the economic uncertainties in rearing halibut, there is reluctance among farmers and lending institutions to risk the larger sums of money needed to purchase halibut juveniles compared to less expensive juvenile salmon. There is a need to complete pre-commercial trials and validate experimental data through a large pilot project.
Juvenile Atlantic halibut (50,000 individuals) of 3 size ranges were transferred into cages in December 2005 for a 3 year (2006- 2009) performance evaluation and disease study. Research will focus on; 1) determining the optimum size for the transfer of juvenile halibut to sea cages for grow-out plus evaluate the consequences of early maturation and sex on growth performance, 2) fish health and vaccine trials using individually tagged fish to evaluate the efficacy and impact of vaccines on growth and survival, 3) production efficiencies and marketing aspects of this flatfish. Environmental monitoring will also be conducted.
Valuable economic, marketing and production data will be collected under commercial and scientific conditions. This is an ideal example of the collaborative commitment by multiple government and academic institutions working together to achieve scientific advancements for sustainable aquaculture development.
Research team: Tillmann Benfey (UNB), D.J. Martin-Robichaud (DFO), Neil Ridler (UNB), Larry Hammell (AVC), Sandi McGeachy (NB DAFA), Skip Wolf (Canadian Halibut Inc.). For information contact D.J. Martin-Robichaud (Email: martin-robichaudd@mar.dfo-mpo.gc.ca). Submitted by DFO (ACRDP).
Jan. '06-Mar. '09
Caption:
Tagging and weighing halibut.
Cod broodstock development through selective breeding

Cod stripping team: Lynn Lush (left), Andy Walsh (center) and Dwight Drover (back).
To further develop Atlantic cod broodstock for the aquaculture industry, selective breeding of cod is being investigated in Atlantic Canada. To date, cod aquaculture has been based on broodstock derived from unselected wild-caught adult cod. The ability to identify and select parent fish which produce offspring exhibiting the most desirable characteristics will further advance the industry by producing the best quality fish possible.
Valued traits such as optimal growth, disease resistance, environmental tolerance, survival, and delayed maturation, if determined to be heritable, can be selected to produce an elite cod broodstock. Difficulties with traits such as these have been some of the limiting factors in the progression of the cod industry throughout Atlantic Canada. It is the aim of the project to help reduce these limitations by developing an elite broodstock.
The current ACRDP funded genetic selection broodstock project in Newfoundland and Labrador focuses on the development of optimal paired mating and egg collection protocols in Atlantic cod, and is working in conjunction with the recently announced Genome Atlantic, Cod Genome Project, funded by Genome Canada. The goal of these co-operating projects is the betterment of the local cod industry through the production of healthy, highly productive cod offspring and elite broodstock
Research team: Lynn Lush (DFO), Jonathan Moir (Northern Cod Ventures), Velmurugu Puvanendran (Memorial U.). For information contact Lynn Lush (Email: lushlp@dfo-mpo.gc.ca). Submitted by DFO(ACRDP).
Apr. '05-Mar. '09
Caption:
Cod stripping team: Lynn Lush (left), Andy Walsh (center) and Dwight Drover (back).
Investigating Nodavirus in Atlantic cod and haddock
This project identified procedures and disinfectants that inactivate the Nodavirus in eggs and water, and examined viral survival in the environment, the vertical and horizontal transmission, and the ages at which cod and haddock are susceptible to infection. The researchers focused on the relationship between spawning stress and virus production in broodstock, and the relationship between stress, immune function and the development of the disease.
The researchers found that all of the nodaviruses were closely related to one another but were distinct from the European isolates already sequenced. Regardless of host species, isolates from close geographical localities were more similar than those from distant geographical areas. At the protein level, differences in coat protein sequences were seen only for strains isolated from Atlantic cod originating from Newfoundland.
These results suggested that NNV may have been present in Atlantic Canada and east coast of USA for some time and evolved to form a monophyletic group, distinct from other isolates found in cold water species. cDNA libraries have been produced and used to clone immune system specific genes important in host-viral interactions.
Research Team: Stewart Johnson (Dalhousie U.), Laura Brown (NRC-IMB); Brian Dixon (U. Waterloo); David Groman (UPEI), Carmencita Yason (AVC); Gilles Olivier (Dalhousie U.). For information contact Stewart Johnson (Email: stewart.johnson@nrc-cnrc.gc.ca). Submitted by AquaNet.
2003 -2006
Newfoundland researchers test new diets for marine finfish in Atlantic Canada

Haddock Broodstock, Sandy Cove Facility, NRC, NS

Cod Broodstock, Aquaculture Research and Development Facility, MUN, NL.
The development of basic production protocols for Eastern Canada’s marine finfish species— cod, haddock, wolfish, and winter flounder —is key for the modernization and diversification of the Canadian aquaculture industry. However, as with many new marine species, the rearing of the larval stages represents a major bottleneck for the mass production of juveniles. Problems related to nutrition of larvae are considered responsible for the majority of the mortality observed during larviculture. This research project focuses on the feeding of larvae and juveniles. Although hatchery feeding technology follows similar protocols worldwide, modifications are necessary to meet species-specific needs.
Research to date has identified better livefeed enrichments by improving lipid-to-protein and essential fatty acid ratios that promote best performance in cod and haddock larvae. The influence of dietary lipids on the stress response of cod larvae has also been investigated. Exposure to stress caused significant differences in larval survival between dietary treatments. Whole body corticosteroid concentrations are now being analysed. In addition, work is in progress to determine the thermal sensitivity of protein synthesis and degradation in juvenile spotted wolfish throughout their development. To do this a new method has been developed for measuring the rate of protein synthesis using mass spectrometry.
Research team: Joe Brown, Alexandre Garcia, Stewart Johnson, Simon Lamarre, Chris Parrish and Sarah Westelmajer. For information contact Chris Parrish (E-mail: cparrish@mun.ca). Submitted by AquaNet. Apr. ’03-May ’06
Researchers optimize lipid utilization in marine finfish diets
Culturing alternate marine finfish species to Atlantic salmon on a commercial scale faces many challenges. While salmon have high muscle lipid deposition, sablefish and halibut have intermediate lipid deposition and cod and haddock have low muscle lipid content. As a result, dietary lipid requirements vary for these different species and need to be tailored accordingly. Signs of fatty acid deficiency include skin lesions, fin erosion, susceptibility to stress, fatty liver and embryonic deformities.
Our research project has generated new information on lipid utilization particularly in regard to essential fatty acid requirements, nutritional values of various lipid sources, and energy utilization for haddock, sablefish, cod and Atlantic halibut. Different experimental diet formulations were tested to examine the effects of partially replacing anchovy oil in grower diets for the preceding fish species with either cold-pressed flaxseed oil, canola oil, and/or poultry fat with respect to fish growth, health and flesh quality responses. Also, attempts were made to ensure that the flesh lipids contained sufficient omega3 (n-3) highly unsaturated fatty acid levels at the "finishing stage" to maintain the human health benefits associated with these important fatty acids.
We also studied the impacts of the different dietary lipid compositions on the basic immune systems of these fish species and made recommendations on the possible implications for fish health management. The research showed that flaxseed and sunflower oil-rich diets caused an increase in the monounsaturated fatty acid content of muscle and other tissues and in some species resulted in fatty liver and related symptoms. Diets containing the alternate lipid sources therefore need be complemented by marine fish oils to increase the polyunsaturated fatty acid content of the fish before they can be marketed and to ensure adequate dietary concentrations of n-3 highly unsaturated fatty acids and arachidonic acid for good growth, health and normal tissue structure.
Research team: Santosh Lall, Dave Higgs, Fereidoon Shahidi, Shannon Balfry, Carla Walbourne, Dulce Alves Martins, Erin Friesen, Duan Zeng, Ying Zhen. For more information contact Santosh Lall (Email: santosh.lall@nrc-cnrc.gc.ca ). Submitted by AquaNet.
Sept. ’03-’06
Immune function, stress and metabolism of haddock and Atlantic cod

Cod fitted with tail differential pressure transducer.

Computer-controlled, and automated, tank respirometry system used to measure the cost of digestion in groups of juvenile Atlantic cod and haddock.
The aim of this research was to enhance growth and reduce disease-related losses at cage sites by: optimizing feeding protocols and diet composition at the cagesites when water temperatures are sub-optimal; understanding how water temperature and aquaculture practices influences stress, metabolism, and immune parameters; and testing the effectiveness of stimulants of the immune system at improving growth and reducing disease occurrence.
Researchers involved in this study conducting feeding (diet), stress and growth trials on cod and haddock at different temperatures, and swim-performance tests on cod to evaluate technologies with potential use in telemetry systems for monitoring metabolism and activity. They also completed DNA sequencing of primary immunological molecules from haddock and cod, tested the effectiveness of several immunostimulant products, and developed and refined effective immunostimulant protocols. The research has identified several immunostimulant products and developed application protocols that appear to be effective in stimulating the immune system and reducing infection rates when exposed to some pathogens (e.g. Loma). It has led to a much better understanding of the stress responses of haddock and cod in relation to husbandry and environmental conditions, and determined that both EMG and ultrasonic tail differential pressure transducers have potential for use in telemetry studies on activity/metabolism in free-swimming gadids.
Research team: Kurt Gamperl, Joe Brown, Duane Barker, Brian Dixon, Stewart Johnson, George Iwama, Scott McKinley, Atef Mansour. For information contact Kurt Gamperl (Email: kgamperl@mun.ca). Submitted by AquaNet.
May ’03-Sept. ’06
Spotted wolffish cultivation: a pilot scale study

Technician Danny Ouellet holds a wolffish.
Private investors and researchers in Canada and Norway have been investigating the potential for spotted wolffish (Anarhichas minor) aquaculture. This project facilitates the collaboration of Canadian and Norwegian researchers with the industry partners in order to address the development of a joint broodstock and nutrition program.
The objectives of this project are to acquire, maintain and manage broodstock populations for future support of R&D and commercial initiatives by increasing juvenile production levels, produce reliable growth and productivity data for North American populations of spotted wolffish, provide economic analyses of spotted wolffish cultivation, and plan pilot scale production operations. The broodstock genetics program includes spotted wolffish acquisition and performance for comparison of Canadian and Norwegian stocks. Juvenile and grow-out trials to commercial size will assess the growth performance of Canadian stocks (versus Norwegian) in relation to temperature and salinity environmental conditions.
Bio-economical research aimed at spotted wolffish cultivation will contribute towards building a knowledge base and likely validate further investments in this commercial activity both by governmental agencies and industrial partners. Price considerations, costs of production and solving important input constraints are important aspects for a successful commercialization. The project will contribute to "best practice" technologies not only for the production of juveniles, but also for the production of the finished product and help establishing realistic estimates of cost by a commercial enterprise.
Research team: James Wilson, Nathalie Le François, Pierre Blier, France Dufresne, Robert Roy, Laura Halfyard. For information contact James Wilson (Email: james_wilson@uqar.qc.ca). Submitted by AquaNet.
Nov. ’04-Mar. ’07
Atlantic cod genomics and broodstock development the focus of East Coast research

The decline in wild cod populations has resulted in fisheries closures throughout Atlantic Canada. Cod aquaculture is recognized as a way to supply cod to the marketplace while providing stability to the established salmon aquaculture industry through species diversification. Broodstock selection is an important aspect of developing a new candidate species for culture. The Atlantic Cod Genomics and Broodstock Development Project (CGP, www.codgene.ca), an $18.1 million project managed by Genome Atlantic, was initiated to create two regional family-based selective breeding programs in New Brunswick/New Hampshire and Newfoundland & Labrador and to develop fundamental cod genomics tools for application within these programs. Cod families will be evaluated and selected for commercially valuable traits such as growth, survival, age of sexual maturation, stress tolerance, disease resistance and product quality and yield.
To date the CGP has produced 107 full and half sibling families for communal rearing in sea cages. Preliminary data analysis has revealed significant variation among families for traits such as juvenile growth. The project has also more than doubled the amount of cod DNA sequence data available in the public domain (3,500 sequences). These sequences will be analysed to help identify DNA markers associated with traits, such as disease resistance and stress tolerance. The CGP also includes social research in intellectual property protection, environmental law, and public consultation.
Research team: Jane Symonds (Huntsman MSC), Sharen Bowman (Atlantic Genome Centre), Keith Culver (UNB), Jake Elliott (Cooke Aquaculture Inc), Kurt Gamperl (OSC MUN), Stewart Johnson (NRC-IMB), Jonathan Moir (Northern Cod Ventures Ltd.), George Nardi (GreatBay Aquaculture), Andy Robinson (U. Guelph), Ed Trippel (DFO). For information contact Jane Symonds (Email: jsymonds@huntsmanmarine.ca; Website: www.codgene.ca). Submitted by Cod Genome Project.
Jan. ’06-Dec. ’09
Flatfish genomics and disease research
Disease caused by viral and bacterial infections limits the production of juvenile marine finfish in many regions including Canada and Spain. Atypical Aeromonas salmonicida and nodavirus infections are cause for concern among the marine finfish aquaculture industries in both of our countries. Within this collaborative project we have developed genomic resources for Atlantic halibut and turbot including expressed sequence tags (ESTs) and cDNA microarrays. These resources have been used to examine their responses to vaccination (halibut) and disease challenge (halibut and turbot). In addition to providing tools for flatfish research this program has allowed for exchanges of graduate students and researchers between the partner institutions and provided opportunities for advance training in the application of molecular biology in fish disease research.
Research team: Stewart Johnson (IMB NRC), Antonio Figueras (CSIS), Kyoung Park (NRC-IMB), Laura Brown (NRC-IMB), Beatriz Novoa (IIM CSIC), José Meseguer Peñalver (Campus de Espinardo), Victoriano Mulero Méndez (Campus de Espinardo). For information contact Stewart Johnson (Email: stewart.johnson@nrc-cnrc.gc.ca). Submitted by NRC-IMB.
Sept. ’04-Mar. ’07
Modeling offshore cage systems for best performance
In 2004, IOT researcher Bruce Colbourne completed a series of model tests of a new surface cage/mooring/feeding system developed by AEG Ltd of New Brunswick. This work was supported by the Industrial Research Assistance Program of NRC. Based on a successful set of experiments, AEG has embarked on a series of full scale prototype evaluation trials. The feeder component of the system was deployed in early 2006 on an existing farm in NB and preparations are underway to conduct full scale trials of the cage and mooring system, in early 2007. The results of the full scale trials will provide AEG with in-situ performance data and will be used by IOT researchers to further develop modelling and scaling techniques.
Research team: Bruce Colbourne. For information contact Bruce Colbourne (Email: D.Colbourne@nrc-cnrc.gc.ca). Submitted by NRC.
Researchers test alternate protein sources for marine finfish
The need for nutritionally balanced, cost-effective diets for development of Atlantic halibut, cod and haddock culture in Atlantic Canada is widely recognized. Our present research at NRC’s Institute for
Marine Biosciences addresses key areas of protein utilization and metabolism by marine fish. Proteins and their constituent amino acids are essential components of aquatic animal diets. Dietary protein requirements of these fish for maximum growth ranges from 45-55 % protein provided sufficient and appropriate amounts of essential amino acids and available energy are supplied.
Although high-quality fish meal is the major source of protein in marine fish feeds, the increasing demands of global aquaculture upon this finite resource necessitates that feeds become increasingly comprised of alternative protein sources of plant and/or animal origin. Incorporation of highly digestible alternative protein sources in marine fish diets must support similar performance to fish meal, make economic sense and, concurrently, have little effect upon fish health and the environment. Measuring nutrient and energy digestibility is a crucial first-step in determining the potential use of alternative protein sources in least-cost ration formulations.
We have conducted studies with halibut, haddock and cod to determine the protein and energy digestibility for a range of feed ingredients widely available in Canada, including fish meals, crustacean by-product meals, animal by-product meals and plant-based feed ingredients (including oilseeds, pulses and cereal grains). The projects have demonstrated that marine species digest nutrients and energy from alternate protein sources relatively well and there is good potential to increase the use of these resources in marine fish diets, especially, meals of crustacean, oilseed and/or cereal grain origin.
Research team: Santosh Lall, Sean Tibbetts, Joyce Milley and Randy Peach. For more information contact Santosh Lall (Email: santosh.lall@nrc-cnrc.gc.ca). Submitted by NRC-IMB.
Apr. ’04-Dec. ’06
Halibut Genomics: An array of opportunities
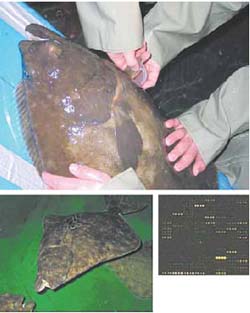
Top: Female halibut being stripped of eggs.
Above left: Adult halibut in tanks.
Above right: Subgrid of halibut microarray.
Atlantic halibut is a promising species for the aquaculture industry in Atlantic Canada. However, significant gains in production can be made by improving our knowledge of the basic biology of this animal and by selective breeding of individuals with desirable traits. This is the focus of the $5.1M Pleurogene project (www.pleurogene.ca) at the National Research Council’s Institute for Marine Biosciences (NRC-IMB). Funded by Genome Canada/Atlantic and Genome Espana, the project aims to enhance flatfish aquaculture using large-scale genomics and proteomics.
Two evolutionarily related, aquaculturally-relevant fish species are under investigation as part of Pleurogene: the cold-water Atlantic halibut and the Mediterranean Senegal sole. Building on the significant efforts in halibut mapping by researchers at DFO St. Andrews, NRC-IMB and Scotian Halibut Ltd., genetic linkage maps are being constructed for both species, and will be used in the selection of improved broodstock. In addition, large-scale sequencing efforts have resulted in the acquisition of approximately 13,000 new halibut and 10,000 new sole sequences, a phenomenal increase in what was previously known. This sequence information has been incorporated into two microarray platforms that will be used to simultaneously monitor changes in the expression of thousands of genes in response to changes in diet, disease, environmental change, stress or any other parameter of interest to producers of fish in intensive culture conditions. Improved understanding of the biological processes underlying fish growth and development will ultimately lead to enhanced production and economic gain.
Research team: Sue Douglas, Michael Reith, Harry Murray, Makoto Matsuoka, Leah Knickle, Darrin Reid, Cheryl Smith and Jennifer Kimball. For more information contact Sue Douglas. (Email: susan.douglas@nrc.ca; Website: www.pleurogene.ca). Submitted by NRC-IMB.
Aug. ’04-Jun. ’07
Comparison of stress responses in finfish
Despite a large number of studies, we still have a poor understanding of how stress affects fish health, reproduction and growth. Furthermore, it is not known whether all species of fish will respond to stress in the same manner. Our research is focused on understanding the effects of stress on physiological and immunological processes of fish.
We are comparing the effects of short (heat shock or handling) and long-term stress (daily handling) on species of interest to the aquaculture industry such as Atlantic salmon, cod and haddock. We have found that there are differences in the stress response between species. Long-term stress suppressed growth in haddock but not in Atlantic salmon. Comparison of the stress hormone levels (cortisol) has shown that Atlantic salmon adapts to daily handling while haddock do not. Cod and haddock, unlike Atlantic salmon did not show increased plasma glucose levels when subjected to different stressors. Cod and haddock also did not increase heat shock protein 70 (hsp70) levels in response to a heat shock.
The relationship between these results and the immune response is being investigated. These studies are showing that species react differently when subjected to the same stressors; demonstrating that husbandry practices in aquaculture operations might have to be fine-tuned to take into consideration the species being raised.
Research team: Luis Afonso, Stewart Johnson, Laura Brown (NRC IMB) and Kurt Gamperl (OSC MUN). For information contact Luis Afonso (Email: luis.afonso@nrc-cnrc.gc.ca). Submitted by NRC-IMB.
Apr. ’04-Mar. ’08
Flatfish antibiotics: novel therapeutants from unlikely sources

Winter flounder (species from which pleurocidin was first isolated).
Fish from Atlantic Canada are not just a delicacy served on a plate – they can also be an important source of novel therapeutants, and in fact coldwater flatfish may prove to be just that. Researchers at NRC’s Institute for Marine Biosciences have used genomics to isolate antimicrobial peptide coding sequences from marine fish with the aim of producing effective low-cost alternatives to increasingly resistance-prone antibiotics.
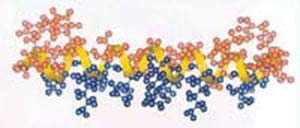
Model of an alpha-helical antimicrobial peptide such as pleurocidin.
First isolated from the disease-resistant winter flounder, these molecules have since been isolated from a wide variety of related flatfish. Synthetic peptides based on the gene sequences have been tested against a diverse spectrum of bacteria as well as a fungus and some have shown potent antimicrobial activity, whereas toxicity against fish and human cells is negligible. In addition to their crucial role in killing microbes, these peptides may play a role in host defense by mobilizing the immune system to ward off infections, heal wounds and even prevent tumor growth.
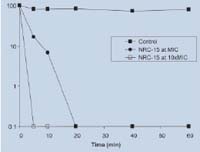
Killing curve of Multiple Resistance Staphylococcus Aureus (MRSA) by pleurocidin NRC-15.
Current research is directed towards the application of these molecules in aquaculture, where they may be of use in treating disease outbreaks in larval fish that are not amenable to vaccination. In addition, preliminary studies are underway to test their potential in human medicine.
Research team: Sue Douglas, Aleks Patrzykat, Jeff Gallant, Harry Murray, Bandi Srinivasulu, Anna Greenshields and Leah Knickle. For more information contact Sue Douglas. (Email: susan.douglas@nrc.ca; Website: http://www.imb.nrc.ca/projects/peptides/index_e.php). Submitted by NRC-IMB.
Shortening the reproductive cycle of the wolffish using photoperiod

A number of studies have shown that manipulating the photoperiod shortens the reproductive cycle in fish and can increase the production of juveniles. One constraint on the development of the wolffish mariculture industry is the lack of a large number of eggs and juveniles. The main objective of this study is to reduce the maturation and spawning cycle of a wolffish population by manipulating the photoperiod. The accelerated maturation is monitored by measuring steroids (estradiol, 11-ketotestosterone) in the plasma of males and females. We will also develop an immunoassay for measuring the egg protein vitellogenin (VTG), a tool for monitoring female gonad development.
The experiments began in January 2006 at the Centre aquacole marin de Grand-Rivière. The manipulation study began with the Atlantic wolffish (Anarhichas lupus) in order to test the method with a sufficient number of spawners. One group is being exposed to a seasonal photoperiod, while the second is being exposed to a shortened seasonal photoperiod of eight months. The experimental tanks have been isolated using photoperiod control tents, equipped with a dawn and dusk simulator. Every month, blood samples are taken and the spawners are examined by ultrasound. Preliminary observations indicate an impact of the photoperiod on the regulation of the reproductive cycle. The morphometric measurements of growth indicate that the oocytes of the experimental groups are more developed than those of the control groups. At the Maurice Lamontagne Institute laboratory, we are continuing work on development of an ELISA for VTG (protein characterization, antibody validation).
Research team: Robert Roy, Nathalie Le François, Bernard Antonin, Dupont Cyr, Domynick Maltais, Robert Vaillancourt. For information contact Robert Roy (Email: royro@dfo-mpo.gc.ca). Submitted by SODIM.
East Coast project measures net drag & added mass
Institute for Ocean Technology (IOT) researchers Bruce Colbourne, Wayne Raman-Nair, Pengfei Liu and Shin Chin, with support from AEG Ltd of New Brunswick and the Canadian Centre for Fisheries Innovation (CCFI), continue working on a project to quantify the added mass and drag characteristics of full scale and small-scale netting.
This year a Memorial University of Newfoundland Graduate student, Cheslav Balash, completed a number of experiments with a new drag measuring apparatus built at IOT. This has allowed the group to more accurately quantify the steady state drag for various types of netting. A first round of experiments has been conducted in waves and techniques to extract the added mass coefficients from this data are being developed. In addition a numerical model has been developed and is being exercised using the experimental data as a reference case. The goal of the project is to have a full set of scaling relationships developed for netting within two years. This will improve confidence in load and motion characteristics predicted from scale model tests for offshore aquaculture systems.
Research team: Bruce Colbourne, Wayne Raman-Nair, Pengfei Liu, Shin Chin and Cheslav Balash. For information contact Bruce Colbourne (Email: D.Colbourne@nrc-cnrc.gc.ca). Submitted by NRC.
- Date modified: