Mikrocytos spp. of Oysters
On this page
Category
Category 2 (in Canada and of regional concern)
Common, generally accepted names of the organism or disease agent
Mikrocytosis, Microcell disease of oyster, Denman Island disease for M. mackini.
Scientific name or taxonomic affiliation
The first enigmatic microcell parasite (Abbott and Meyer 2014) to be named was Mikrocytos mackini in Crassostrea gigas from British Columbia, Canada (Farley et al. 1988). Since 1988 2 new species were described, Mikrocytos boweri in Ostrea lurida and Crassostrea gigas also from British Columbia, Canada (Abbott et al. 2014) and Mikrocytos mimicus in Crassostrea gigas from Norfolk coast, UK. (Hartikainen et al. 2014). Initial genetic analysis suggested that M. mackini may be a basal eukaryote that is not closely related to other known protistan taxa (Carnegie et al. 2003, Abbott et al. 2012). With the advent of next-generation sequencing methods, Burki et al. (2013) presented robust evidence that M. mackini belongs to the eukaryotic supergroup Rhizaria and that it most likely harbors a reduced mitochondrion-related organelle. This interpretation was supported by several investigators (van der Giezen 2013, Abbott 2014, Abbott and Meyer 2014, Carnegie and Engelsma 2014). Hartikainen et al. (2014) proposed a new family (Mikrocytiidae) and new order (Mikrocytida) within the class Ascetosporea (Rhizaria: Cercozoa) for Mikrocytos spp. and Paramikrocytos canceri a sister linage that they described from the European edible crab (Cancer pagurus) (Hartikainen et al. 2014). Also in oysters, genetically divergent Mikrocytos-like protists were reported in Ostrea lurida (= conchaphila) in San Francisco Bay, California, USA (Friedman et al. 2005), from the North Atlantic Ocean in Ostrea edulis from Atlantic Canada that had been imported into France for research purposes (Gagné et al. 2008), in C. gigas from the northern coast of the Yellow Sea, China (Wang et al. 2010) and in C. gigas from a single location (Lemmen's Inlet) on the west coast of Vancouver Island, British Columbia, Canada (Abbott et al. 2011). In addition to oysters, microcells have been reported in clams. Mikrocytos veneroïdes and Mikrocytos donaxi were described in wedge clams Donax trunculus from France (Garcia et al. 2012, 2018) and a Mikrocytos–like parasite was reported from Manila clams Venerupis (= Ruditapes) philippinarum on the coast of Galicia, Spain (Ramilo et al. 2014). Hartikainen et al. (2014) detected Mikrocytos DNA sequence in a sample of mixed copepod species collected from the South Atlantic.
Additional research into the Mikrocytos-like protists is required to understand the taxonomic relationships between species of Mikrocytos (Abbott et al. 2012). Abbott and Meyer (2014) suggest that minimally new species descriptions for Mikrocytos incorporate 18S-rDNA sequence data as well as histopathological and host information. They also indicated that the inclusion of electron microscopy information in taxonomic descriptions of Mikrocytos is desirable but not feasible when the prevalence and intensity of infections are low (e.g. Gagné et al. 2008; Wang et al. 2010; Abbott et al. 2011, 2014). Note that Mikrocytos spp. are not related to microcells in the genus Bonamia (e.g., B. ostreae , B. exitiosa , B. roughleyi and other Bonamia spp.) which are now known to be within the sister taxon Haplosporidia of the Rhizaria (Carnegie and Cochennec-Laureau 2004, Abbott et al. 2012, Hartikainen et al. 2014, Abbott et al. 2014, Abbott and Meyer 2014).
Geographic distribution
Protists positively identified as Mikrocytos mackini are known to occur in Crassostrea gigas on the Canadian west coast, probably ubiquitous throughout the Strait of Georgia and confined to other specific localities around Vancouver Island, and adjacent areas of the State of Washington, USA (Elston et al. 2015). Recently, M. mackini was detected in Crassostrea sikamea from Humboldt Bay, California, USA and the DNA sequence of the complete internal transcribed spacer (ITS) region of the ribosomal DNA (i.e., ITS1-5.8S-ITS2 array) was 100% homologous with the published M. mackini sequence (Elston et al. 2012). Abbott et al. (2011) hypothesized that the total lack of genetic variation within M. mackini across the complete ITS1-5.8S-ITS2 region in over 70 samples collected throughout its known range on the south coast of British Columbia and Puget Sound, Washington, could be a result of a founder effect if the parasite had been introduced into the west coast of North America alongside C. gigas, which was imported from the east coast of Asia beginning around 1914 to about 1961. In the original description of M. mackini, Farley et al. (1988) indicated that a closely related candidate for inclusion within this species was found in C. gigas from Hawaii, USA in September 1972. Hill et al. (2014) reported that an archival sample originally identified as Mikrocytos mackini in C. gigas from Kaneohe Bay, Hawaii, from 1972 (Farley et al. 1988) hybridized to generic probes for Bonamia spp. when assayed via in situ hybridization using digoxigenin-labeled probes.
Mikrocytos boweri was described from 2 native (wild) Ostrea lurida collected from Swy-A-Lana Lagoon in Nanaimo on the east coast of Vancouver Island, British Columbia, Canada (Abbott et al. 2014). A conspecific microcell was identified in C. gigas from a single location (Lemmen's Inlet) on the west coast of Vancouver Island, British Columbia, Canada (Abbott et al. 2011, 2014).
Mikrocytos mimicus was described from a population of farmed C. gigas near Brancaster on the north Norfolk coast, United Kingdom (Hartikainen et al. 2014). Mikrocytos mimicus was also observed by histopathology and DNA sequencing in C. gigas displaying 90% mortality in May near the island Schiermonnikoog, Wadden Sea, in the Netherlands (ICES 2018).
Friedman et al. (2005) reported a Mikrocytos-like protist from Ostrea lurida (= conchaphila) in San Francisco Bay, California, USA. Other isolates of Mikrocytos-like protist were reported from the North Atlantic Ocean in Ostrea edulis from Atlantic Canada that had been imported into France for research purposes (Gagné et al. 2008) and in C. gigas from the northern coast of the Yellow Sea, China (Wang et al. 2010).
Host species
Mikrocytos mackini was reported in Crassostrea gigas, Ostrea lurida (previously called Ostrea (= Ostreola) conchaphila which is now known to be a separate species (Polson et al. 2009) of unknown susceptibility to M. mackini) and Crassostrea sikamea and can experimentally infect Crassostrea virginica and Ostrea edulis (Bower et al. 1997, Elston et al. 2012). Laboratory bath exposure experiments clearly indicated that juvenile C. gigas were susceptible to infection with M. mackini while juvenile Panope abrupta (geoduck clams) and mature Venerupis philippinarum (Manila clams about 28 mm in shell length) were resistant (Bower et al. 2005 and Meyer et al. 2008, respectively).
Mikrocytos boweri was reported in O. lurida and C. gigas (Abbott et al. 2014) and M. mimicus was reported only in C. gigas (Hartikainen et al. 2014).
Mikrocytos-like protozoa in oysters were reported in O. lurida (= conchaphila) from San Francisco Bay, California, USA (Friedman et al. 2005), in O. edulis from Atlantic Canada that had been imported into France for research purposes (Gagné et al. 2008), and in C. gigas from the northern coast of the Yellow Sea, China (Wang et al. 2010).
Impact on the host
Focal intracellular infection of vesicular connective tissue cells which results in haemocyte infiltration and tissue necrosis. The resulting greenish pustules can reduce the marketability of diseased oysters. Severe infections of M. mackini appear to be restricted to older oysters (over 2 years) and mortalities (often about 30% of older oysters at low tide levels) occur predominantly in April and May after a 3-4 month period when temperatures are less than 10 °C. Following the development of sensitive and specific molecular detection techniques (Polinski et al. 2015), Polinski et al. (2017) confirmed the potential for extracellular seawater transmission of M. mackini and also identified host gill to have the highest early and continued prevalence for M. mackini DNA compared to stomach, mantle, labial palps or adductor muscle samples. However, under laboratory conditions, infections following waterborne challenge were slow to develop despite a substantial exposure (>106 M. mackini per litre for 24 hours) and further investigation demonstrated that M. mackini occurrence and infectivity severely declined following extracellular seawater incubation of more than 24 hours (Polinski et al. 2017).
Approximately 10% of C. gigas affected by M. mackini appear to recover. Crassostrea gigas seems to be more resistant to the disease caused by M.mackini than the other species of oysters challenged experimentally under laboratory and field conditions (Bower et al. 1997). In the State of Washington, USA, mortalities attributed to M. mackini have not been encountered (Elston et al. 2015).
Only low prevalence of M. boweri (2 of 40 oysters in April and 0 of 60 in July of 2012), with no gross signs of disease, were observed in O. lurida (Abbott et al. 2014). Similar low prevalence of infection without evidence of gross pathology was reported for M. boweri (initially identified as Mikrocytos sp.-BC) in C. gigas (Abbott et al. 2011, Abbott and Meyer 2014). However, diffuse and focal haemocyte infiltration consistent with light intensity infections of intracellular Mikrocytos parasites (<50 M. boweri detected per tissue section) were observed by histology (Abbott et al. 2014).
Mikrocytos mimicus was associated with a mortality event (about 20% of the oysters dead or dying) in a population of farmed C. gigas, held at an intertidal shellfish farm (Hartikainen et al. 2014). Affected oysters were considered to be thin and watery with obvious green pustules associated predominately with the surface of the mantle tissue and adductor muscle. Twelve out of 35 of the affected stock examined histologically had M. mimicus associated with intense focal haemocyte infiltration and areas of necrosis in the connective tissues (Hartikainen et al. 2014 Supplemental Results).
Risk assessment and risk management strategies in regard to the hazard presented by M. mackini were formulated during a workshop coordinated by R. E. Elston and conducted by the Pacific Shellfish Institute, Tacoma, Washington, USA in 2004. The workshop found that there was negligible risk of exporting M. mackini with live shellfish products in the states of Washington, Oregon and California. However, the apparent increased pathogenicity of M. mackini and/or increased susceptibility to disease of oysters at a cool temperatures suggest that M. mackini poses a risk to oysters cultured in waters with extended periods (several months) at temperatures less than 10 °C. The report of M. mackini in C. sikamea from Humboldt Bay, California revealed a higher prevalence of infection in oysters with a longer residence time in the bay and from locations with somewhat colder than typical winter seawater temperatures (Elston et al. 2012). Abbott and Meyer (2014) presented information that indicates the temperature dependence of M. mackini pathology occurs across the genus, such that clinical infections induced by Mikrocytos species are generally dependent on cool water temperatures.
Diagnostic techniques
Gross observations
Focal lesions (ulcerations, abscesses, pustules usually green in colour but can be yellow-brown or colourless) up to 5 mm in diameter, within the body wall, adductor muscle or on the surfaces of the labial palps or mantle. Often there is a brown scar on the shell, adjacent to abscess on the mantle surface. However, lesions alone cannot be used to confirm that a Mikrocytos is the causative agent. For example, the green pustules resulting from nocardiosis are indistinguishable from those caused by M. mackini (Bower and Meyer 2004). Note that C. gigas infected with M. mackini tend to appear in prime condition (i.e., with an overall smooth, cream-coloured, rounded body and thick, crinkly, mantles, see Figures 1 to 3 below). However, C. gigas infected with M. mimicus were considered to be thin and watery (Hartikainen et al. 2014 Supplemental Results).


Figure 1 and 1a. Crassostrea gigas removed from shell and illustrating lesions (arrows) characteristic of Mikrocytos mackini when the microcell is most abundant in the vesicular connective tissue cells immediately surrounding the lesions.

Figure 1b. Three lesions (arrows) characteristic of M. mackini on the labial palps of C. gigas.
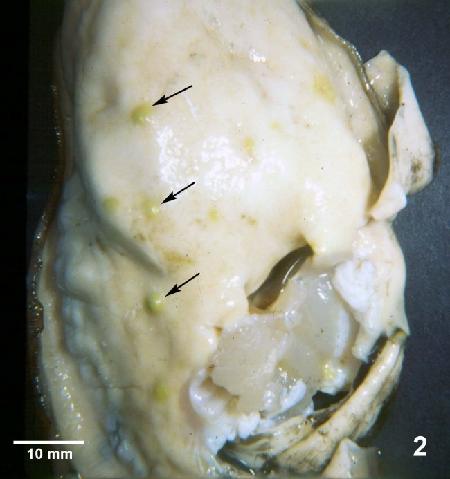
Figure 2. Crassostrea gigas removed from shell and illustrating lesions (arrows) observed during later stages of Denman Island disease. Typically, Mikrocytos mackini can no longer be found in oysters by histology at this advanced stage of the disease.

Figure 3. Ostrea edulis, with top valve removed, illustrating numerous lesions in the adductor muscle (arrow) caused by Mikrocytos mackini.

Figure 3a. Crassostrea gigas removed from shell and illustrating lesions in the adductor muscle (arrows) caused by M. mackini.

Figure 3b and 3c. Adductor muscles of C. gigas with cryptic lesions (3b, arrows) and very pronounced lesions (3c, arrows) caused by M. mackini.
Histology
High power (1000x oil immersion magnification) microscopic examination of vesicular connective tissue cells immediately adjacent to foci of intense haemocyte infiltration (lesions) for the presence of intracellular protozoa 2-3 µm in diameter. Mikrocytos spp. have also been observed in muscle cells and occasionally in haemocytes within the lesions. Mikrocytos spp. tend to be spherical in shape. The nucleus of M. mackini and M. boweri are often located near the center of the cell and spherical in shape (S. M. Bower, personal observations; Abbott et al. 2014) whereas, the nucleus of M. mimicus is normally eccentric and often elongated when examined histologically and as tissue imprints (Hartikainen et al. 2014 Supplemental Results). Note that the small size and intracellular habit of M. mackini combined with its faint staining characteristics when using routine histological techniques often make its microscopic detection difficult (Meyer et al. 2005). Also, the small cell size and limited characteristic morphological features makes distinguishing among Mikrocytos species and confidently separating them from Bonamia microcells not possible by light microscopy (Abbott and Meyer 2014). The only other species initially included in the Mikrocytos genus but now known to be unrelated, Bonamia (= Microcytos) roughleyi which causes Australian winter disease in Saccostrea commercialis, differs from M. mackini by having a cytoplasmic vacuole. No such vacuole was reported in either Mikrocytos spp. or other Bonamia spp.

Figure 4. Histological section through a lesion caused by Mikrocytos mackini on the mantle of Crassostrea gigas. This intracellular protozoan (not visible at this magnification) usually occurs in the intact vesicular connective tissue cells immediately surrounding the periphery of the lesion (arrows). Haematoxylin and eosin stain.
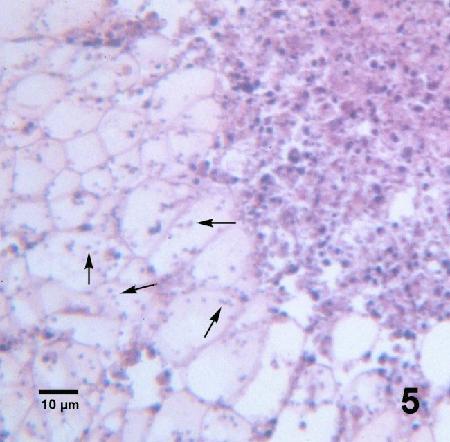
Figure 5. Many Mikrocytos mackini (arrows) within vesicular connective tissue cells adjacent to a lesion characterized by an accumulation of haemocytes and necrotic cells. Haematoxylin and eosin stain.

Figure 6. Oil immersion magnification (1000x magnification) of Mikrocytos mackini (arrows) within the cytoplasm of vesicular connective tissue cells of Crassostrea gigas. Haematoxylin and eosin stain.

Figure 7. As for Fig. 6 but from a different Crassostrea gigas. Because of the small size of M. mackini, it is very difficult to visualise and photograph in histological preparations. Haematoxylin and eosin stain.

Figure 8. Mikrocytos mackini (A) within fibres of the adductor muscle of Crassostrea gigas. One M. mackini is located close to the nucleus (B) of a muscle cell. Haematoxylin and eosin stain.
Tissue imprints
Imprints of lesions are air dried, fixed and stained as for Bonamia ostreae in oyster tissue smears and examined using 1000x magnification (oil immersion) for the small microcells that are often observed free of the host cells. In such preparations, it is not possible to differentiate between M. Mikrocytos spp. and Bonamia spp. In tissue imprints, M. mimicus were generally ovoid to spherical, measuring 3-5 μm in diameter with basophilic cytoplasm and an eosinophilic nucleus. Nuclei were mainly subcentral or peripheral and variable in shape, ranging from spherical through to ovoid as well as occasionally pyriform (Hartikainen et al. 2014 Supplemental Results).

Figure 9. Mikrocytos mackini (arrows) among host cell debris in a tissue imprint from a lesion in the adductor muscle of a laboratory infected Crassostrea gigas. Hemacolor® stain.
Electron microscopy
Ultrastructural morphology differentiates Mikrocytos spp. from Bonamia spp.; the nucleolus of Mikrocytos spp. are located towards the centre of the nucleus while that of B. ostreae has an eccentric location and there are no haplosporosomes and no canonical mitochondria in Mikrocytos spp. (Hine et al. 2001). However, Burki et al. (2013) sequenced part of the transcriptome of M. mackini and discovered 4 mitochondrion-derived genes in M. mackini indicating that it harbours a yet-to-be described, double-bounded, reduced mitochondrion-related organelle (Abbott and Meyer 2014). The cell ultrastructure of M. mimicus (Hartikainen et al. 2014) was found to be highly similar to that of M. mackini.

Figure 10.Electron micrograph of a Crassostrea gigas vesicular connective tissue cell containing Mikrocytos mackini (arrows). Uranyl acetate and lead citrate stain.
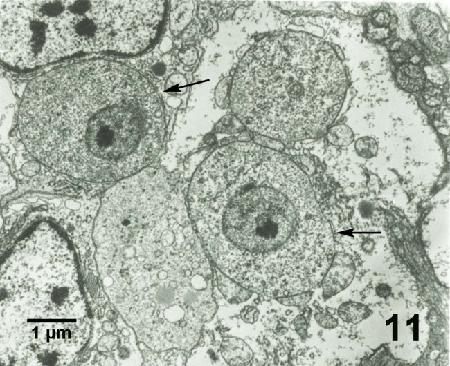
Figure 11. Mikrocytos mackini (arrows) each containing a nucleus with a pronounced nucleolus and lacking mitochondria. Uranyl acetate and lead citrate stain.
Detailed studies on the ultrastructure of M. mackini identified 3 morphological forms (Hine et al. 2001). These 3 morphological forms were also reported for M. mimicus (Hartikainen et al. 2014 Supplemental Results). The quiescent cells (QC) had a central round to ovoid nucleus, less than 7 cisternae of inactive nuclear membrane-bound Golgi, few vesicles and lysosome-like bodies. They occurred in the vesicular connective tissue cells, haemocytes (hyalinocytes), adductor and heart myocytes and extracellularly. The vesicular cells (VC) contained many small coated and uncoated vesicles, lacked nuclear membrane-bound Golgi-like arrays and the nuclear membrane was sometimes dilated to form a cisternal chamber. They were rarely extracellular and usually occurred in adductor and heart myocytes, in close association with host cell mitochondria. The endosomal cells (EC) had a dilated nuclear membrane, a well-developed anastomosing endoplasmic reticulum connected the nuclear and plasma membranes and endosomes were present in the cytoplasm. They occurred in the vesicular connective tissue cells, haemocytes (hyalinocytes), and extracellularly. Few organelles occurred in all forms of M. mackini and may be due to obligate parasitism and the utilization of host cell organelles, thus reducing the need for parasite organelles.
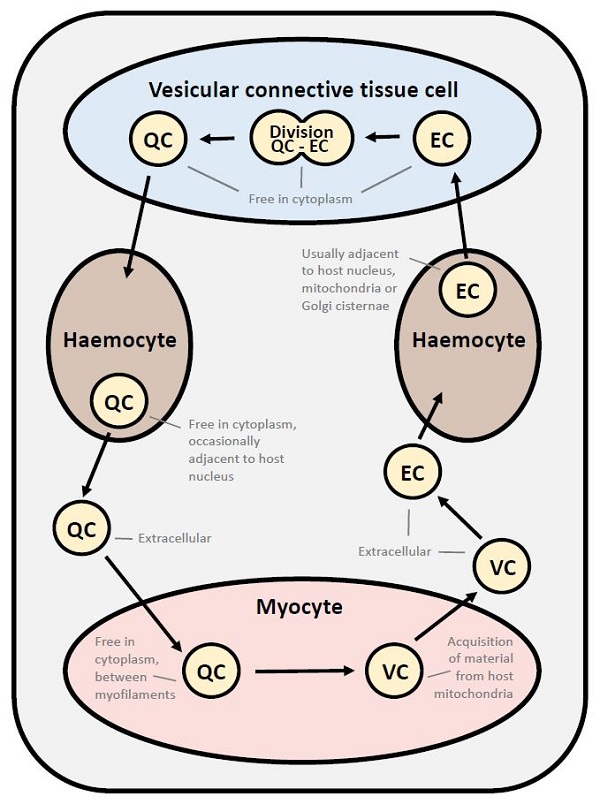
Figure 12. Proposed developmental cycle of Mikrocytos mackini indicating host cell type and host organelle affiliation for the 3 recognized morphological forms consisting of quiescent cell (QC), vesicular cell (VC) and edosomal cell (EC).
Immunological assay
Hybridomas that produce monoclonal antibodies specific for M. mackini have been produced (S. M. Bower) and can be made available for the development of diagnostic tool(s) similar to that developed for Bonamia ostreae (ELISA, IFA).
DNA probes
Polymerase chain reaction (PCR) and fluorescent in situ hybridization (FISH) assays were developed based on a 1457 base pair segment of the small-subunit ribosomal DNA (SSU rDNA or 18S) gene (GenBank accession number AF477623). In part, the molecular analysis of M. mackini was made possible by an enrichment method that concentrated the microcells in a 1.5 ml Eppendorf tube after several steps of filtration to remove much of the host tissue (Joly et al. 2001). The primers that were initially used to identify M. mackini DNA were shown to preferentially amplify various parasitic protistan SSU rDNA from metazoan tissues via PCR (Bower et al. 2004). A more specifically designed M. mackini PCR detected 3 to 4 times more M. mackini infections than standard histopathology (Carnegie et al. 2003). However, the PCR assay cross-reacted with the Mikrocytos-like protist from the Atlantic Ocean, Yellow Sea, and west coast of Vancouver Island (Gagné et al. 2008, Wang et al. 2010, and Abbott et al. 2011, respectively) and with M. boweri (Abbott and Meyer 2014). Although the segment of the Mikrocytos-like protist that was amplified represented a short portion (about 500 base pairs) of the 18S gene, it was surprisingly exactly the same (100%) in samples from O. edulis in the Atlantic Ocean and C. gigas from the Yellow Sea and west coast of Vancouver Island but about 10 to 20% divergent from that of M. mackini (Gagné et al. 2008; Wang et al. 2010; Abbott et al. 2011, 2014; Abbott and Meyer 2014). As recommended by Abbott et al. (2011), sequencing a longer portion of rDNA including both the 18S gene and the more quickly evolving ITS regions was found to be informative and used for the characterization of Mikrocytos spp. Differences in the 18S gene sequences were used to identify M. boweri and M. mimicus, important criteria because morphology-based discrimination is limited with Mikrocytos spp. (Abbott et al. 2014, Hartikainen et al. 2014 Supplemental Results). The entire length of the internal transcribed spacer regions (ITS1 and ITS2) and the intervening 5.8S gene of rDNA of M. mackini was sequenced (GenBank accession number HM563060). This sequence was found to contain regions of high divergence (in ITS1 and ITS2) from the Mikrocytos-like protist (now known as M. boweri, see Abbott et al. 2014) from British Columbia that could be exploited for differential molecular diagnostics (Abbott et al. 2011). This same gene region (i.e., the complete ITS1-5.8S-ITS2) was surprisingly homologous (100% identical) among numerous M. mackini isolates from throughout its known range (Abbott et al. 2011, Elston et al. 2012).
A TaqMan quantitative PCR (qPCR) assay targeting the ITS2-28S that is specific to M. mackini was developed and statistical analysis showed that a mid-body slice of oyster tissue was optimal for detecting the parasite (Lowe et al. 2012). Subsequently, a species-specific, sensitive, and quantitative method for detecting M. mackini DNA from host tissues using probe-based real-time qPCR technology was developed and proved to be superior to existing methods based on conventional PCR, histology or gross pathology and is the first species-specific diagnostic test for M. mackini (Polinski et al. 2015). Additional assessment determined that this qPCR assay had the highest diagnostic sensitivity while maintaining similar diagnostic specificity to both conventional PCR and histopathology and thus is generally well-suited to most applications (Polinski et al. 2021). This method was used to detect M. mackini in seawater and confirmed the potential for extracellular seawater transmission of this parasite (Polinski et al. 2017).
Carnegie et al. (2003) developed the first in situ hybridization (ISH) method which used fluorescently-labelled probes for the detection of M. mackini by hybridizing to the 18S rDNA. Later, Meyer et al. (2005) used digoxigenin-labelling of 1 of the DNA probes designed by Carnegie et al. (2003) and found this in situ hybridization method (DIG-ISH) allowed for easy detection of M. mackini at low magnification with much higher sensitivity than routine histology. This DIG-ISH was used to reveal M. mackini in digestive gland tissues, an organ not previously known to be inhabited by this parasite (Meyer et al. 2005).
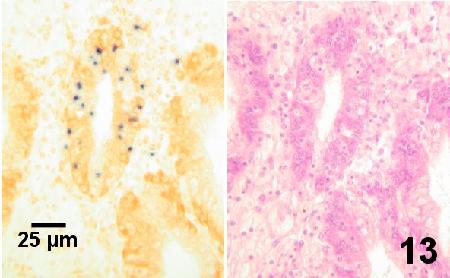
Figure 13. Consecutive sections of the same area of the digestive gland of an adult Crassostrea gigas infected with Mikrocytos mackini (dark spots on left). The section on the left was stained with a digoxigenin-labelled DNA probe (DIG-ISH) and revealed the parasite within the tubule at relatively low magnifications. Parasites were not discernable in similar tissues stained with haematoxylin and eosin stain (section on right) even when examined at high magnification (1000x - oil emersion objective).
The in situ hybridization (ISH) probe for M. mackini cross-reacted with the Mikrocytos-like protist from the Atlantic Ocean and Yellow Sea (Gagné et al. 2008, Wang et al. 2010, respectively) but not with the Mikrocytos-like protist from San Francisco Bay (Friedman et al. 2005). Also, the ISH assay developed for the Mikrocytos-like protist was not specific such that the Mikrocytos-like probe hybridized to both M. mackini and the Mikrocytos-like protist (Gagné et al. 2008).
Methods of control
Oysters from infected areas (currently or historically) should not be moved to areas where Denman Island disease has not been recorded especially if the areas have a cooler water temperature profile. Within the southern half of British Columbia where the disease is known to occur, the effect of the disease on infected populations can be reduced to a manageable level by harvesting or moving large oysters to locations high in the intertidal zone prior to March and not planting oysters at lower tide levels before June (Quayle 1982, 1988). Oysters retained over a 2-year oyster production cycle, within areas where the disease is active, can experience in the following spring increased mortalities and the development of obvious lesions (Fig. 2 above) that reduces marketability.
Laboratory studies by Polinski et al. (2017) confirmed that seawater transmission of M. mackini between Pacific oysters is possible and that M. mackini DNA is naturally
shed from living oysters during infection. However, the low infection efficiency and short environmental stability of M. mackini would geographically restrict the effectiveness for
this mode of transmission in natural environs. Exposure studies have also demonstrated
that M. mackini can persist for long periods once inside the host (Hervio et al. 1996, Polinski et al. 2017). Thus, modest amounts of seawater transmission in conjunction with long-term persistence could be the cause of the sporadic localized areas where Denman Island disease is currently observed. This information is important to consider when assessing biosecurity and diagnostic screening measures with relation to M. mackini (Polinski et al. 2017). Nevertheless, the application of recently developed sensitive and specific diagnostic assays should significantly assist the development of disease management programs (Polinski et al. 2015, 2021).
References
Abbott, C.L. 2014. Evolution: hidden at the end of a very long branch. Current Biology 24: 807-812.
Abbott, C.L. and G.R. Meyer. 2014. Review of Mikrocytos microcell parasites at the dawn of a new age of scientific discovery. Diseases of Aquatic Organisms 110: 25-32.
Abbott, C.L., S.R. Gilmore, G. Lowe, G. Meyer and S. Bower. 2011. Sequence homogeneity of internal transcribed spacer rDNA in Mikrocytos mackini and detection of Mikrocytos sp. in a new location. Diseases of Aquatic Organisms 93: 243–250.
Abbott, C.L., N. Corradi, G. Meyer, F. Burki, S.C. Johnson and P. Keeling. 2012. Multiple gene segments isolated by next-generation sequencing indicate extreme divergence of Mikrocytos mackini. Journal of Shellfish Research 31: 257. (Abstract).
Abbott, C.L., G.R. Meyer, G. Lowe, E. Kim and S.C. Johnson. 2014. Molecular taxonomy of Mikrocytos boweri sp. nov. from Olympia oysters Ostrea lurida in British Columbia, Canada. Diseases of Aquatic Organisms 110: 65-70.
Bower, S.M. 1988. Circumvention of mortalities caused by Denman Island Oyster Disease during mariculture of Pacific Oysters. American Fisheries Society Special Publication 18: 246-248.
Bower, S.M. 2003. Mikrocytosis (Mikrocytos mackini). Annual Reports of OIE Reference Laboratories and Collaborating Centres 2002: 403-405.
Bower, S.M. 2001. Hazards and risk management of Mikrocytos mackini in oysters. In: Rodgers, C.J. (eds), Proceedings of the OIE International Conference on Risk anaysis in aquatic animal health. World Organisation for Animal Health, Paris, pp. 164-166.
Bower, S.M. 2005. Mikrocytos mackini (microcell). In: Rohde, K. (ed.), Marine Parasitology. CSIRO Publishing, Collingwood, pp. 34-37.
Bower, S. M. and G. R. Meyer. 1999. Effects of cold water on limiting or exacerbating some oyster diseases. Journal of Shellfish Research 18: 296. (Abstract).
Bower, S.M. and G.R. Meyer. 2004. 5.2.5 Mikrocytosis (Denman Island Disease of Oysters), In: AFS-FHS (American Fisheries Society-Fish Health Section). 2014. FHS blue book: suggested procedures for the detection and identification of certain finfish and shellfish pathogens, 2020 edition.
Bower S.M., S.E. McGladdery, and I.M. Price. 1994. Synopsis of infectious diseases and parasites of commercially exploited shellfish. Annual Review of Fish Diseases 4: 36–39.
Bower, S. M., D. Hervio and G. R. Meyer. 1997. Infectivity of Mikrocytos mackini, the causative agent of Denman Island disease in Pacific oysters Crassostrea gigas, to various species of oysters. Diseases of Aquatic Organisms 29: 111-116.
Bower, S.M., R.B. Carnegie, B. Goh, S.R.M. Jones, G.J. Lowe and M.W.S. Mak. 2004. Preferential PCR amplification of parasitic protistan small subunit rDNA from metazoan tissues. The Journal of Eukaryotic Microbiology 51: 325-332.
Bower, S.M., K. Bate and G.R. Meyer. 2005. Susceptibility of juvenile Crassostrea gigas and resistance of Panope abrupta to Mikrocytos mackini. Journal of Invertebrate Pathology 88: 95-99.
Burki, F., N. Corradi, R. Sierra, J. Pawlowski, G.R. Meyer, C.L. Abbott and P.J. Keeling. 2013. Phylogenomics of the intracellular parasite Mikrocytos mackini reveals evidence for a mitosome in Rhizaria. Current Biology 23: 1541-1547.
Carnegie, R.B. and N. Cochennec-Laureau. 2004. Microcell parasites of oysters: recent insights and future trends. Aquatic Living Resources 17: 519-528.
Carnegie, R.B., G.R. Meyer, J. Blackbourn, N. Cochennec-Laureau, F.C.J. Berthe and S.M. Bower. 2003. Molecular detection of the oyster parasite Mikrocytos mackini and a preliminary phylogenetic analysis. Diseases of Aquatic Organisms 54: 219-227.
Elston, R.A. 1993. Infectious diseases of the Pacific oyster, Crassostrea gigas. Annual Review of Fish Diseases 3: 259-276.
Elston, R.A., J. Moore and C.L. Abbott. 2012. Denman Island disease (causative agent Mikrocytos mackini) in a new host, Kumamoto oysters Crassostrea sikamea. Diseases of Aquatic Organisms 102: 65-71.
Elston, R., C. Friedman, L. Gustafson, G. Meyer and R. Rogers. 2015. Denman Island disease in Washington State, USA: distribution and prevalence in Pacific and Olympia oysters. Diseases of Aquatic Organisms 114: 147-154.
Farley, C.A., P.H. Wolf and R.A. Elston. 1988. A long-term study of "microcell" disease in oysters with a description of a new genus, Mikrocytos (g.n.) and two new species Mikrocytos mackini (sp.n.) and Mikrocytos roughleyi (sp.n.). U.S. National Marine Fish Service Bulletin 86: 581-593.
Friedman, C.S., H.M. Brown, T.W. Ewing, F.J. Griffin and G.N. Cherr. 2005. Pilot study of the Olympia oyster Ostrea conchaphila in the San Francisco Bay estuary: description and distribution of diseases. Diseases of Aquatic Organisms 65: 1-8.
Garcia, C., I. Arzul, J.P. Joly, B. Guichard, B. Chollet, E. Omnes, C. Haond, M. Robert, C. Lupo and C. Francois. 2012. Mikrocytos like protozoans and the shellfish Donax trunculus mortality events in France. Journal of Shellfish Research 31: 273. (Abstract).
Garcia, C., C. Haond, B. Chollet, M. Nerac, E. Omnes, J.-P. Joly, C. Dubreuil, D. Serpin, A. Langlade, D. Le Gal, A. Terre-Terrillon, O. Courtois, B. Guichard and I. Arzul. 2018. Descriptions of Mikrocytos veneroïdes n. sp. and Mikrocytos donaxi n. sp. (Ascetosporea: Mikrocytida: Mikrocytiidae), detected during important mortality events of the wedge clam Donax trunculus Linnaeus (Veneroida: Donacidae), in France between 2008 and 2011. Parasites & Vectors 11: 119, 16 pp.
Gagné, N., N. Cochennec, M. Stephenson, S. S. McGladdery, G.R. Meyer and S.M. Bower. 2008. First report of a Mikrocytos-like parasite in European oysters Ostrea edulis from Canada after transport and quarantine in France. Diseases of Aquatic Organisms 80: 27-35.
Hartikainen, H., Grant D. Stentiford, Kelly S. Bateman, C. Berney, Stephen W. Feist, M. Longshaw, B. Okamura, D. Stone, G. Ward, C. Wood and D. Bass. 2014. Mikrocytids are a broadly distributed and divergent radiation of parasites in aquatic invertebrates. Current Biology 24: 807-812 and Supplemental Information.
Hervio, D., S. M. Bower and G. R. Meyer. 1995a. Life cycle, distribution and lack of host specificity of Mikrocytos mackini, the cause of Denman Island disease in Pacific oysters, Crassostrea gigas. Journal of Shellfish Research 14: 228. (Abstract)
Hervio, D., G. R. Meyer, S. M. Bower and R. D. Adlard. 1995b. Development of specific molecular probes for serological and PCR assays for the identification and diagnosis of Mikrocytos mackini, the cause of Denman Island disease in the Pacific oyster Crassostrea gigas. Journal of Shellfish Research 14: 268. (Abstract).
Hervio, D., S.M. Bower and G.R. Meyer. 1996. Detection, isolation and experimental transmission of Mikrocytos mackini, a microcell parasite of Pacific oysters Crassostrea gigas (Thunberg). Journal of Invertebrate Pathology 67: 72-79.
Hill, K.M., N.A. Stokes, S.C. Webb, P.M. Hine, M.A. Kroeck, J.D. Moore, M.S. Morley, K.S. Reece, E.M. Burreson and R.B. Carnegie. 2014. Phylogenetics of Bonamia parasites based on small subunit and internal transcribed spacer region ribosomal DNA sequence data. Diseases of Aquatic Organisms 110: 33-54.
Hine, P.M., S.M. Bower, G.R. Meyer, N. Cochennec-Laureau and F.C.J. Berthe. 2001. Ultrastructure of Mikrocytos mackini, the cause of Denman Island disease in oysters Crassostrea spp. and Ostrea spp. in British Columbia, Canada. Diseases of Aquatic Organisms 45: 215-227.
ICES. 2018. Report of the Working Group on Pathology and Diseases of Marine Organisms (WGPDMO), 13-17 February 2018, Riga, Latvia. ICES CM 2018/ASG:01. p. 42 pp. (see page 9).
Joly, J.-P., S.M. Bower and G.R. Meyer. 2001. A simple technique to concentrate the protozoan Mikrocytos mackini, causative agent of Denman Island disease in oysters. The Journal of Parasitology 87: 432-434.
Lowe, G., G. Meyer, M.G. Abbott, S.C. Johnson and C.L. Abbott. 2012. Development of a q-PCR assay to detect Mikrocytos mackini and assessment of optimum tissue for diagnostic testing. Journal of Shellfish Research 31: 315. (Abstract).
Meyer, G.R., S.M. Bower and R.B. Carnegie. 2005. Sensitivity of a digoxigenin-labelled DNA probe in detecting Mikrocytos mackini, causative agent of Denman Island disease (mikrocytosis) in oysters. Journal of Invertebrate Pathology 88: 89-94.
Meyer, G.R., S.M. Bower, G. Lowe and S. Davies. 2008. Resistance of the Manila clam (Venerupis philippinarum) to infection with Mikrocytos mackini. Journal of Invertebrate Pathology 98: 54-57.
Polinski, M., G. Lowe, G. Meyer, S. Corbeil, A. Colling, C. Caraguel and C.L. Abbott. 2015. Molecular detection of Mikrocytos mackini in Pacific oysters using quantitative PCR. Molecular and Biochemical Parasitology 200: 19-24.
Polinski, M.P., G.R. Meyer, G.J. Lowe and C.L. Abbott. 2017. Seawater detection and biological assessments regarding transmission of the oyster parasite Mikrocytos mackini using qPCR. Diseases of Aquatic Organisms 126: 143-153.
Polinski, M.P., E. Laurin, M.K.V.C. Delphino, G.J. Lowe, G.R. Meyer and C.L. Abbott. 2021. Evaluation of histopathology, PCR, and qPCR to detect Mikrocytos mackini in oysters Crassostrea gigas using Bayesian latent class analysis. Diseases of Aquatic Organisms 144: 21-31.
Polson, M.P., W.E. Hewson, D.J. Eernisse, P.K. Baker and D.C. Zacherl. 2009. You Say Conchaphila, I Say Lurida: Molecular Evidence for Restricting the Olympia Oyster (Ostrea lurida Carpenter 1864) to Temperate Western North America. Journal of Shellfish Research 28: 11-21.
Quayle, D.B. 1961. Denman Island disease and mortality, 1960. Fisheries Research Board of Canada Manuscript Report 713. Ottawa.
Quayle, D.B. 1969. Pacific oyster culture in British Columbia. Fisheries Research Board of Canada Bulletin 169: 192 p. (For information on Denman disease see pages 166-168).
Quayle, D.B. 1982. Denman Island oyster disease 1960-1980. British Columbia Shellfish Mariculture Newsletter 2(2): 1-5. (Victoria, Canada).
Quayle, D.B. 1988. Pacific oyster culture in British Columbia. Canadian Bulletin of Fisheries and Aquatic Sciences 218: 241 p. (For information on Denman disease see pages 115-117).
Ramilo, A., D. Iglesias, E. Abollo, M. González, S. Darriba and A. Villalba. 2014. Infection of Manila clams Ruditapes philippinarum from Galicia (NW Spain) with a Mikrocytos-like parasite. Diseases of Aquatic Organisms 110: 71-79.
van der Giezen, M. 2013. Evolution: one thread to unite them all. Current Biology 23: R679-R681.
Wang, Z., Y. Liang and X. Lu. 2010. Use of histopathology, PCR and in situ hybridization methods to detect the parasite Mikrocytos sp. in Pacific oyster Crassostrea gigas from the northern coast of the Yellow Sea, China. Aquatic Living Resources 23: 125-130.
Citation information
Bower, S.M. (2021): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Mikrocytos spp. of Oysters.
Date last revised: February 2021
Comments to Susan Bower
- Date modified: