Bonamia ostreae of Oysters
On this page
Category
Category 2 (In Canada and of Regional Concern)
Common, generally accepted names of the organism or disease agent
Microcell disease, Bonamiasis, Bonamiosis, Haemocyte disease of flat oyster, Haemocytic parasitosis.
Scientific name or taxonomic affiliation
Bonamia ostreae (Pichot et al. 1980). Hill et al. (2010a) indicated that the genetic variability between different “isolates” of B. ostreae is considered limited and currently no strains of B. ostreae are identified. Results of initial ultrastructural studies suggested that this protist was affiliated with the Haplosporidia despite the lack of a spore stage (Bonami et al. 1985, Brehélin et al. 1982). This taxonomic affiliation was subsequently confirmed by DNA analysis (Carnegie et al. 2000b, Reece et al. 2004, López-Flores et al. 2007, Hill et al. 2010a, Hartikainen et al. 2014). Engelsma et al. (2014) indicated that the genus Bonamia represents a derived clade within the phylum Haplosporidia whose members have generally adopted (1) life cycles based on direct oyster to oyster transmission of uninucleate amoeboid cell forms, and (2) intracellular infection of oyster haemocytes by these cell forms. Related species include Bonamia exitiosa originally described as a pathogen of New Zealand dredge oysters Ostrea chilensis, Bonamia (=Mikrocytos) roughleyi a pathogen of Sydney rock oysters Saccostrea glomerata, Bonamia perspora a parasite of the crested or horse oyster Ostreola equestris and other unidentified Bonamia spp. from various species of oysters in distant locations.
Geographic distribution
Western Europe along the coast from Spain to Netherlands, Ireland and the United Kingdom (England: Cornwall and Isles of Scilly, Devon, Dorset, Somerset, Essex, Hampshire and Isle of Wight, Kent, West Sussex, Wales: South West Wales, Scotland: Highlands and Islands (Engelsma and Hine 2009b, Laing et al. 2014)). ICES (2004, page 16) indicated that B. ostreae has also been detected in Denmark. Bonamis ostreae was reported for the first time from Morocco (Laayoune province) in 2005 (Culloty and Mulcahy 2007). It was detected in archived (in 1990) samples of Ostrea edulis from the Manfredonia Gulf (Adriatic Sea) of Italy and again detected there along with Bonamia exitiosa in a few (3 of 750) O. edulis collected in 2007 (Narcisi et al. 2010). Concurrent infections with B. exitiosa also occur in Galicia, northwest Spain (Ramilo et al. 2014). Earliest records were from the west (California and Washington) and east (Maine) coasts of the USA. In California during the mid 1960s, Katkansky and Manzer (1967) reported high mortalities and heavy infections of microcells in O. edulis that had originated from Milford, Connecticut a few years earlier. (Note that one archived sample of infected O. edulis from Milford that was identified using histology and electron microscopy as B. ostreae by Farley et al. (1988) was subsequently determined to be B. exitiosa by Hill et al. (2014) using molecular analysis. Bonamia sp. that is closely related to Bonamia exitiosa was also detected in O. edulis from California (Hill et al. 2014)) In both Washington and Maine, the prevalence of infection is usually low and heavy infections are rare. Current evidence suggests that B. ostreae was inadvertently introduced into Maine, Washington and Europe from California by the translocation of infected O. edulis in the late 1970s (Elston et al. 1986, Friedman and Perkins 1994, Cigarría and Elston 1997, Saulnier et al. 2007). The introduction and spread of B. ostreae within Europe has foremost been linked to transfers of shellfish (Peeler et al. 2010), either directly or, for example, via other anthropogenic routes such as hull fouling (Howard 1994, Culloty and Mulcahy 2007, Engelsma et al. 2014). In the fall of 2004, this parasite was detected for the first time in O. edulis farmed in British Columbia, Canada (Marty et al. 2006).
Host species
Originally described from Ostrea edulis and according to Hill et al. (2014), B. ostreae has only been detected in O. edulis. However, B. ostreae has been found in Crassostrea ariakensis (=rivularis) by histology and electron microscopy and identity later confirmed by DNA sequencing (Cochennec et al. 1998, Audemard et al. 2005, Engelsma and Hine 2009b, Engelsma et al. 2014). This parasite has also been reported but not confirmed (i.e., host species for which incomplete or unclear data prevent a clear conclusion) from Ostrea chilensis (=Tiostrea chilensis, =Tiostrea lutaria, =Ostrea lutaria) (Grizel et al. 1982), Ostrea angasi (Bougrier et al. 1986), Ostrea puelchana (Pascual et al. 1991), Ostrea denselamellosa (Carnegie and Cochennec-Laureau 2004) and Crassostrea angulata (Katkansky et al. 1969). Confirmation of species identity is required because Bonamia exitiosa is now known to overlap the distribution of B. ostreae on the coast of Europe (Engelsma et al. 2014). The Pacific oyster, Crassostrea gigas (Renault et al 1995, Cao et al. 2009, Lynch et al. 2010), mussels, Mytilus edulis and Mytilus galloprovincialis, and clams, Ruditapes decussatus and Venerupis (=Ruditapes) philippinarum could not be naturally nor experimentally infected and these bivalves did not appear to act as vectors nor intermediate hosts for the parasite (Culloty et al. 1999). However, C. gigas may act as a carrier or reservoir host of B. ostreae as indicated by Lynch et al. (2010) who reported detecting positive Polymerase chain reaction (PCR) signal and visualized a few B. ostreae-like cells in haemocytes and extracellularly in two C. gigas. Microcells in the vesicular connective tissue cells of Ostrea conchaphila (=Ostrea lurida) from Oregon, USA were speculated to be B. ostreae (Farley et al. 1988). However, Elston (1990) indicated that although experiments suggest that O. conchaphila may contract the disease, infection has not been positively demonstrated and Arzul et al. (2005a) could not infect O. conchaphila by cohabitation for 11 months with diseased O. edulis.
Impact on the host
Thebault et al. (2003) listed and applied 24 epidemiological and experimental criteria to demonstrate that B. ostreae was the infectious agent responsible for mass mortalities of O. edulis. Bonamia ostreae, in conjunction with earlier epizootics caused by Martelia refringens, caused a drastic drop in the French production of O. edulis from 20,000 t per year in the 1970's to 1,800 t in 1995 (Boudry et al. 1996; Arzul et al. 2005b, 2006). Bonamia ostreae has also had a significant negative impact on O. edulis production throughout its distribution range in Europe (Tigé et al. 1981, 1982; Grizel 1983, Culloty and Mulcahy 2007). Although many infected oysters appear normal, others may have yellow discolouration and/or extensive lesions (i.e. perforated ulcers) in the connective tissues of the gills, mantle and digestive gland. Pathology appears correlated to haemocyte destruction and diapedesis due to proliferation of B. ostreae (Balouet et al. 1983, Berthe 2004). The transmission of infection between oysters is direct with no requirement for an intermediate host (Tigé et al. 1982, Tigé and Grizel 1982, Poder et al. 1983, Hervio et al. 1995, Culloty et al. 1999) and this has been experimentally demonstrated in the field as well as by cohabitation and injection in the laboratory (Bachère et al. 1986, Lallias et al. 2008). However, the possible involvement of a carrier/reservoir host should not be ruled out (Lynch et al. 2006, 2010). When benthic macroinvertebrates and zooplankton from a B. ostreae-endemic area were screened for the presence of parasite DNA, using polymerase chain reaction (PCR), 8 benthic macroinvertebrates and 19 grouped zooplankton samples gave positive results, and in the laboratory the transmission of B. ostreae was effected to two naïve O. edulis cohabiting in the laboratory with the brittle star, Ophiothrix fragilis (Lynch et al. 2007). Although these positive results with alternate hosts could be indicative of parasitism, it is equally as plausible that the animals were only casually associated with B. ostreae or had consumed infected oysters (Culloty and Mulcahy 2008).
Within the host oyster, B. ostreae proliferates by mitosis in the branchial epithelial cells and haemocytes (Montes et al. 1994). Infection was demonstrated to result in the increase in the number of tissue infiltrating haemocytes (granulocytic reaction) (Balouet and Poder 1985, Cochennec-Laureau et al. 2003). Although some flat oysters die with light infections, others succumb to much heavier infections. Heavily infected oysters tend to be in poorer condition than uninfected oysters. In one study, the presence of Bonamia was better related to size than to age of O. edulis and infection level was statistically independent of gonadal development stage (Cáceres-Martínez et al. 1995). In another study, prevalence was highest in the largest oysters in spring and declined disproportionately in autumn, possibly due to high mortality of large oysters before autumn, suggesting that prevalence depends on oyster age (Engelsma et al. 2010). However, Robert et al. (1991) and Culloty and Mulchy (1996) found that two years appeared to be the critical age for disease development in O. edulis in the Bay of Arcachon, France and on the south coast of Ireland, respectively. Nevertheless, both 0+ and 1+ year-old O. edulis are susceptible to infection and can develop a high prevalence and intensity of infection over a six-month period with associated mortalities (Lynch et al. 2005a, b; Lallias et al. 2008). Arzul et al. (2011) demonstrated that larvae of O. edulis can be infected with B. ostreae in the epithelium surrounding their visceral cavities while being held within the pallial cavity of infected mother oysters. Larvae might thus contribute to the spread of the parasite during their planktonic life. Some studies reported a seasonal pattern of prevalence and mortality, with highest levels occurring in autumn-winter (Montes 1990, Van Banning 1991, Culloty and Mulcahy 1996, Laing et al. 2014). In France, transmission occurred throughout the year but rates of infection seemed to be less from July to November (15% prevalence compared to 50% prevalence during March to June) (Tigé and Grizel 1982). Also, in marine Lake Grevelingen in the Netherlands and in Ireland (Clew Bay, Lough Foyle and Cork Harbour), B. ostreae was detected in flat oysters throughout the year with a higher prevalence in spring than in autumn (Engelsma et al. (2010), Flannery et al. (2014a), and Lynch et al. (2014), respectively). Male and female oysters were equally affected (Culloty and Mulchy 1996).
In vitro tests were used to determine that haemocytes of C. gigas were able to bind more B. ostreae than were haemocytes of O. edulis (Fisher 1988). This difference in the ability of haemocytes to bind the parasite in conjunction with the apparent inability of O. edulis haemocytes to digest the parasites once they are ingested (Balouet et al. 1983, Chagot et al. 1989, Hervio et al. 1989, Chagot et al. 1992, Xue and Renault 2000) may be factors relevant to the differences in susceptibility to infection and disease development in the two species of oysters. Also, significant differences associated with total and differential haemocyte count, and respiratory burst between O. edulis and C. gigas could be linked to differences in susceptibility to bonamiosis between both species (Comesaña et al. 2012). Cochennec-Laureau et al. (2003) reported that the proportion of granulocytes (granulated haemocytes) in O. edulis decreased with infection possibly as a result of these cells being destroyed or degranulated by B. ostreae suggesting that hyalinocytes (agranular haemocytes) may be involved in parasite survival and/or development. Da Silva et al. (2008) also found a similar correlation and supported the hypothesis that a high percentage of granulocytes and low percentage of hyalinocytes in a stock of O. edulis would enhance oyster immune ability and, consequently, would contribute to lower susceptibility to disease and longer lifespan. In agreement, Comesaña et al. (2012) reported significant changes in total and differential haemocyte counts, and respiratory bursts in O. edulis associated with B. ostreae infections. Cronin et al. (2001) found insignificant correlation between haemolymph protein concentration and lysozyme levels and infection of O. edulis by B. ostreae. In an attempt to understand the molecular basis of the immune response of O. edulis against bonamiosis, Martín-Gómez et al. (2012) used a combination of suppression subtractive hybridization and quantitative-polymerase chain reaction (qPCR) approaches to identify genes involved in the development of responses to infection both in early and advanced phases of the disease caused by B. ostreae and/or Bonamia exitiosa. They determined that the expression of a number of genes related with signal transduction, oxidative stress, chaperones and with leukotriene synthesis or inflammation in O. edulis haemocytes changed in association with bonamiosis (Martín-Gómez et al. 2012). From the results of in vitro experiments, Morga et al. (2009) suggested that B. ostreae actively contributed to the modification of haemocyte activities (decrease in non-specific esterase activities and reactive oxygen species production) in order to ensure its own intracellular survival. Essentially, the parasite is partly able to turn off the metabolic destructive capacity of the haemocytes even though infected oysters and more particularly their haemocytes developed mechanisms to degrade the parasite (Engelsma et al. 2014). Hervio et al. (1991) detected acid phosphatase activity in membrane-bound organelles known as ‘dense bodies' of B. ostreae that may be used to coat and protect the plasma membrane and in this way may contribute to the intracellular survival of the parasite (Engelsma et al. 2014). Also, cytosolic heat shock protein 90 (HSP90) of B. ostreae may play a role in haemocyte invasion (Prado-Alvarez et al. 2013).
Quantitative trait loci (QTL) analyses using a two-stage testing strategy and interval mapping methods were used to detect resistance to B. ostreae in a family of O. edulis derived from a cross between a wild oyster and an individual from a family selected for resistance to bonamiosis (Lallias et al. 2009). Utilizing a proteomic approach, Cao et al. (2009) envisaged the application of two-dimensional electrophoresis to the analysis of haemolymph proteins to understand the interaction between oysters and B. ostreae and to find the bases of tolerance/resistance to bonamiosis. Morga et al. (2010) studied the haemocyte response of O. edulis to B. ostreae at the transcriptome levels based on the use of real time PCR assays and suggested using a combination of glyceraldehyde 3-phosphate-dehydrogenase (GAPDH) and elongation factor 1 alpha (EF1-α) as reference genes (for which they characterized the complete open reading frame (ORF)) when examining expression levels of housekeeping genes in haemocytes of O. edulis. Morga et al. (2011) also used suppression subtractive hybridisation (SSH) to identify five oyster genes (omega glutathione Stransferase (OGST), superoxide dismutase (SOD), tissue inhibitor of metalloproteinase (TIMP), galectin, interferon regulatory factor (IRF-like) and filamin genes) with increased expression in haemocytes infected with B. ostreae. The expressed sequence tags (ESTs) of interest includes genes involved in cytoskeleton, respiratory chain, detoxification membrane receptors, and immune system. Morga et al. (2012) also contributed to a better understanding of the molecular basis involved in the resistance of O. edulis to B. ostreae infection.
Diagnostic techniques
Histology is generally the recommended technique for surveillance in regions only infected by B. ostreae. The recommended methods for presumptive diagnosis of infection with B. ostreae are, for reasons of availability, utility and diagnostic specificity and sensitivity, tissue imprints and a Polymerase chain reaction (PCR). For confirmatory diagnosis, transmission electron microscopy (in some circumstances) and genetic sequencing are recommended (Engelsma and Hine 2009a). Engelsma et al. (2014) presented a summary and discussion of the various diagnostic techniques to detect Bonamia spp.
Gross Observations
Bonamiosis is sometimes accompanied by yellow discoloration and extensive lesions on the gills and mantle of O. edulis infected with B. ostreae. However, most of the infected oysters appear normal (Arzul and Joly 2011; Engelsma and Hine 2009a).
Tissue Imprint (“Heart Smears”)
Make acetone- (or methanol-) fixed impression smears from gill or heart tissue (preferably the ventricle since the auricles contain an abundance of serous cells which make detection of the parasite difficult). Stain with Wright, Wright-Giemsa, May-Grunwald-Giemsa or equivalent stain (e.g., Hemacolor, Merck; Diff-QuiK, Baxter). Examine for 2-5 µm spherical or ovoid organisms with a central nucleus within or outside the haemocytes (Moore 2006, Arzul and Joly 2011). (Note: the organisms are enlarged by this method compared to those in fresh or histological preparations.) This method will also detect B. ostreae in hearts of oysters frozen and stored at -20 °C for at least four years and held at 4 °C for several hours before testing (Rogan et al. 1991). O'Neill et al. (1998) recommended that the ventricular heart smear technique be used in conjunction with either stained haemolymph smears (histocytology) or histology to increase the possibility of detecting light infections. Culloty et al. (2003) indicated that the stained heart smear technique is not reliable for detecting latent infections. Lynch et al. (2008) claimed that heart smear examination was the most sensitive individual technique compared to histology, a polymerase chain reaction (PCR) test and an in situ hybridization (ISH) assay, but a greater sensitivity of detection was obtained when results of heart smear and PCR screening were combined. Results of interlaboratory comparison tests organised by the European Union Reference Laboratory for Molluscan Diseases (IFREMER, La Tremblade) in 2007, 2009 and 2012 (with 20, 18 and 21 participating laboratories, respectively) revealed general competency in both tissue imprint and histological techniques but with more infections detected by the former method (Engelsma et al. 2014). Flannery et al. (2014b) also indicated that heart imprints and histology had the highest reproducibility amongst three separate laboratories that examined four methods of diagnosis: heart imprints, histology, PCR and ISH. However, both heart imprints and histology as well as electron microscopy lack the specificity to identify the species of Bonamia detected.

Figure 1. Bonamia ostreae within haemocytes (arrows) and extracellular (arrow heads) in a heart imprint from a heavily infected Ostrea edulis. Hemacolor stain.
Histocytology: Haemolymph is withdrawn from the adductor muscle into an anticoagulent using a syringe and needle (21 Gauge). The haemocytes are placed (by cytocentrifugation or cell adhesion) in a monolayer onto poly-L-lysine coated glass slides and stained and examined as for tissue imprints. Zabaleta and Barber (1996) observed that results obtained from the examination of stained haemolymph smears and histological preparations of infected O. edulis populations were the same but suggested that histology was preferred for detecting light infections. Da Silva and Villalba (2004) found this technique to be more sensitive in detecting B. ostreae than tissue imprints and histology.
Histology
Examine haematoxylin and eosin stained tissue cross-sections for tiny protozoa (2-4 µm in diameter) within haemocytes (Moore 2006). Bonamia ostreae is distributed systemically in advanced infections (Balouet and Poder 1985). In early infections, B. ostreae are often observed within haemocytes, associated with dense focal haemocyte infiltrations in the connective tissue of the gill and mantle, and in the vascular sinuses around the stomach and intestine (Bucke 1988). Bachère et al. (1982a) preferred stained imprints of gill tissue over histological examination of the digestive gland for the diagnosis of B. ostreae. Arzul and Joly (2011) indicated that histopathology appears more reliable than tissue imprints for the detection of the parasite in case of low level of infections. However, tissue imprints are more rapid and less expensive than histopathology. Van Banning (1990) proposed that B. ostreae was an ovarian tissue parasite for part of its life cycle.

Figure 2a. Bonamia ostreae (arrows) within a few haemocytes in haemal spaces within the connective tissue of the mantle of Ostrea edulis. Haematoxylin and eosin stain.
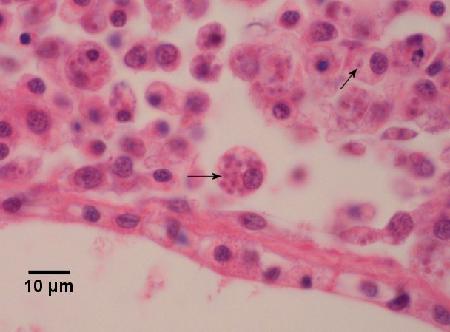
Figure 2b. Bonamia ostreae (arrows) within a few haemocytes in haemal spaces within the connective tissue of the mantle of Ostrea edulis. Haematoxylin and eosin stain.
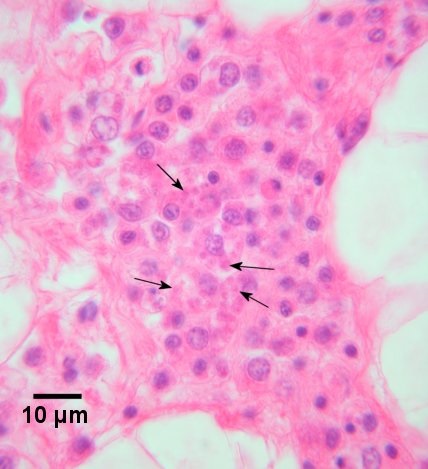
Figure 3. Bonamia ostreae (arrows) in haemocytes within an accumulation of haemocytes in the connective tissue of a heavily infected Ostrea edulis. Haematoxylin and eosin stain.
Electron Microscopy
Prepare tissues according to standard procedures for electron microscopy (e.g., Moore 2006). Uninucleate, diplocaryotic and plasmodial stages with 3 to 5 nuclei have been described and illustrated (Pichot et al. 1980, Comps et al. 1980, Brehélin et al. 1982, Balouet et al. 1983, Bonami et al. 1985, Montes et al. 1994). Intracellular structures include mitochondria, haplosporosomes, Golgi apparatus and persistent intranuclear microtubules. Two forms of B. ostreae were described: dense forms, 2-3 µm in diameter with cytoplasm rich in ribosomes, haplosporosomes and one or two mitochondria; and clear forms, 2-4 µm in diameter with a large nucleolus in the nucleus (Grizel 1987, Bucke 1988). This parasite is usually found within haemocytes. However, Montes et al. (1994) also observed B. ostreae within branchial epithelial cells of O. edulis. Dinamani et al. (1987) compared the Bonamia sp. from Ostrea chilensis (=Tiostrea lutaria) with B. ostreae. Although Hine et al. (2001) and Hill et al. (2010a) presented ultrastructural differences between B. ostreae and B. exitiosa, Narcisi et al. (2010) found that the ultrastructural characteristics of B. exitiosa occurring in Italy were so variable that they cannot be used to definitively identify a Bonamia species. Transmission electron microscopy is not recommended as a diagnostic technique because it is time consuming and not practical for routine application but is recommended when Bonamia-like parasites are described in a new host species (Arzul and Jolu 2011).
Immunological Assay
An immunofluorescent technique based on monoclonal antibodies was developed (Mialhe et al. 1988b, Boulo et al. 1989). However, this technique gave unclear results when tested extensively on oysters from Maine, USA (Zabaleta and Barber 1996). Although direct monoclonal antibody sandwich immunoassay for the detection of B. ostreae in haemolymph samples of O. edulis was developed (Cochennec et al. 1992) and marketed commercially for a few years in the mid 1990s, it is no longer available on the market.
DNA Probes
Molecular procedures for the analysis of samples for Bonamia spp. were described by Moore (2006). Segments of the ribosomal RNA locus (including parts of the small subunit (SSU rDNA or 18S rDNA) and internal transcribed spacers (ITS1)) and two actin genes have been sequenced by polymerase chain reaction (PCR) and molecular cloning (López-Flores et al. 2007; Hill et al. 2010a, Hill et al. 2014). A PCR reaction specific for a rDNA region (528 base pairs (bp) spanning 341 bp of 18S rDNA and 187 bp of ITS1) with a gene sequence resembling that belonging to members of the Phylum Haplosporidia was identified and found to detect the parasite in naturally infected O. edulis in Maine, USA (Carnegie et al. 2000a, b). An in situ hybridization (ISH) assay (Carnegie et al. 1999, 2001, 2003) targeting this region has also been developed. The PCR assay proved to be more sensitive and less ambiguous than standard cytological (tissue imprint) techniques (Carnegie et al. 2000b, Carnegie and Cochennec-Laureau 2004, Lynch et al. 2005b) and histology (Balseiro et al. 2006). Another PCR assay, that amplifies a 300 bp product, was identified from the same area of the genome by Cochennec et al. (2000). In addition to detecting B. ostreae, these assays also detected Bonamia exitiosa and Haplosporidium nelsoni but B. ostreae can be differentiated from the other Haplosporidia by the application of restriction fragment length polymorphism (RFLP) analysis (Hine et al. 2001, a standard operating procedure for this technique is presented at EURL for Molluscs Diseases). Under certain circumstances, the primers of Cochennec et al. (2000) generate a non-specific product of approximately the same size as the expected product of 300 bp (Engelsma and Hine 2009a, Carrasco et al. 2012, Engelsma et al. 2014). The non-specific fragment of 295 bp that can be generated is presumably from host origin making sequencing of the amplicon to confirm the positive results obligatory (Engelsma and Hine 2009a). Another conventional PCR assay was designed by Hill et al. (2010b, 2014) to amplify a ~205 bp region of the SSU rDNA with the widest possible Bonamia spp. specificity, and will amplify all known lineages/species.
Marty et al. (2006) developed a real-time TaqMan® PCR assay that targeted a 68-bp section of the 18S rDNA and was designed not to amplify DNA of other Haplosporidia. This assay proved to have greater diagnostic sensitivity than histopathology even when used to analyse paraffin sections (Marty et al. 2006). Corbeil et al. (2006) also developed a real-time TaqMan® PCR assay for the detection of Bonamia spp. (but not Haplosporidium nelsoni nor Haplosporidium costale) that was comparable to conventional PCR in sensitivity but produced more rapid results with a low risk of sample cross-contamination and which can be optimised to determine the intensity of infection. The real-time PCR assay using SYBR® Green chemistry developed by Robert et al. (2009) did not cross-react with closely related parasites, including B. exitiosa, was at least 10-fold more sensitive than conventional PCR (performed according to Cochennec et al. (2000)) and was quantitative. Ramilo et al. (2013) described species-specific conventional PCR (cPCR) and real-time PCR diagnostic assays for B. ostreae and B. exitiosa in O. edulis as well as a multiplex PCR method to detect both parasites in a single assay. The sensitivity of these procedures was higher using oyster gills and gonad tissue, rather than gills alone. Although the implementation of statistical tools (maximum likelihood method) for the comparison of these assays and histology showed the possibility of false positives, all procedures showed negative results when used for the analysis of oysters from a Bonamia-free area (Ramilo et al. 2013).
Flannery et al. (2014b) determined that PCR had the highest detection level in every laboratory during a study set up in three separate laboratories (one each in Ireland, England and Spain) to examine four, routinely used, methods of diagnosis: heart imprints, histology, PCR and ISH. If PCR is used to detect infection beyond the know geographic and host range of B. ostreae, visualization of the parasite and/or sequencing the product is required for diagnosis and to confirm that the DNA detected by PCR is that of B. ostreae (Culloty and Mulcahy 2007, Narcisi et al. 2010, Engelsma et al. 2014). In general, DNA based diagnosic tools need validation, specificity definition and further development prior to full implementation (Renault 2008). Nevertheless, ISH with a digoxigenin (DIG) labelled probe has been employed to locate light infections of B. ostreae within histological sections of the gills and epithelium of the digestive tract suggesting that these tissues may be the sites of first infection. Hill et al. (2014) described a DIG ISH probe that was specific for B. ostreae. Also, fluorescent ISH using a cocktail of 3 fluorescein labeled probes did not cross-react with H. nelsoni (Carnegie et al. 2003).
Other components of the B. ostreae genome have been described but to date, none of these have been developed into diagnostic assays (Prado-Alvarez et al. 2013).
Culture
Limited multiplication of B. ostreae from explants of gills from heavily infected oysters was achieved after 3 days in vitro at 20 °C (Comps 1983). Protocols for the preparation of purified B. ostreae cell suspensions from infected oysters have been described using a discontinuous density gradient of Percoll (Bachère et al. 1982b, Bachère et al. 1986) and a discontinuous sucrose gradient (Mialhe et al. 1988) The purified cells from both techniques retained infectivity and ultrastructural morphology and have been used in cytochemistry assays of the parasite (Hervio et al. 1991). Purified isolates have also been used to determine that in vitro, B. ostreae had a significantly lower survival at 25 °C compared to 4 °C and 15 °C (especially after 48 hours of incubation), and high salinities (greater than or equal to 35 grams per litre salt in seabed borewater supplemented with natural salt) favoured parasite survival (Arzul et al. 2009).
Methods of Control
Effective management of the disease caused by B. ostreae is complicated by the extensive nature of the oyster production process and limited options for disease control of the cultured stocks in open water surrounded by wild oyster populations (Engelsma et al. 2014). Pathogen transfers via movements of aquatic organisms appear to be a major cause of epizootics (Renault 2008, Engelsma et al. 2014). Some oysters from endemic areas may be asymptomatic and show no sign of Bonamia using routine detection techniques. Because larvae of O. edulis can be infected with B. ostreae while being held within the pallial cavity of infected mother oysters, the transfer of larvae for aquaculture purpose should be controlled especially when they are exported from areas where B. ostreae is present (Arzul et al. 2011). Although no diagnostic method is 100% accurate in the detection of B. ostreae, Flannery et al. (2014b) indicated that the use of both a microscopy based method, to allow for visualisation of the parasite, and a molecular method, to increase sensitivity in low infections, would allow for a more precise diagnosis of B. ostreae and that particular caution is required when screening light (possibly recently acquired) infections. If infected animals are introduced into a naïve population, high mortalities can be expected for at least 6 years (van Banning 1985, 1991). To date, there are no known eradication procedures. Despite early attempts to eradicate B. ostreae from the Netherlands (Van Banning 1988), this parasite is now endemic to O. edulis in marine Lake Grevelingen, the Netherlands (Engelsma et al. 2010).
Mortalities due to bonamiasis can be reduced using suspension culture, reduced handling stress and lower stocking densities (Tigé et al. 1984). In Galicia, Spain, raft cultured oysters suspended at 1-2 meters depth had lower prevalence of infection and fewer mortalities then cohorts held at 8-9 meters depth suggesting that proximity to the sea floor may be a factor in transmission (Lama and Montes 1993). Subtidal growing areas also appear to be less severely affected than intertidal areas. Oyster seed from natural settlement should be avoided because these oysters tend to be significantly more parasitized than seed produced by hatcheries (Conchas et al. 2003). Montes et al. (2003) observed that O. edulis could be successfully cultured in areas of Galicia, Spain, contaminated with B. ostreae if they were promptly marketed after about 15 to 18 months of culture. Also, Arzul et al. (2006) indicated that bonamiosis kills oysters older than two years of age but O. edulis can reproduce after year one. Thus, oyster stocks that are regularly harvested for further growth or marketing results in the elimination of highly infected oysters.
Le Bec et al. (1991) suggested that culturing O. edulis with C. gigas, which are not naturally susceptible to infection, may help to reduce infection in O. edulis. However, in one study, the growth of O. edulis was reduced when they were cultured with C. gigas (Robert et al. 1991). In another study, the mixed rearing of O. edulis and C. gigas did not significantly reduce the prevalence of B. ostreae in O. edulis (Bodoy et al. 1991). Also, B. ostreae may weaken the competitive ability of O. edulis relative to the introduced Pacific oyster C. gigas, particularly in years with high water temperatures (Engelsma et al. 2010). Despite management practices of reducing stocking densities under suspension culture or selling oysters at a lower weight before significant B. ostreae-induced mortalities occur, the production of O. edulis in Europe has remained low due to bonamiosis (Lallias et al. 2008).
Experimental infection by inoculation of B. ostreae into O. edulis from three separate populations in France found no significant difference in susceptibility between the populations (Bachère and Grizel 1983). However, field studies to investigate the potential disease resistance in a number of O. edulis populations from various locations in Europe indicated that some stocks performed significantly better (determined by prevalence and intensity of infection measurements and cumulative mortality) in some trials than others (Culloty et al. 2004). In Quiberon Bay, France where commercial production of O. edulis depends on the transfer of oysters from other regions of Brittany prior to marketing, despite the risks related to transfers of live molluscs, and where B. ostreae has been detected since 1980, the prevalence of B. ostreae is usually lower than 15% with less severe outbreaks than in the past suggesting that the oysters have developed a relative natural tolerance to the parasite (Arzul et al. 2005b). Also, detection frequencies recorded in the two main grow-out areas of France (Quiberon and Cancale bays) were not significantly correlated suggesting that environmental parameters and aquaculture practices have more impact on the evolution of the disease than initial parasite burden (Arzul et al. 2006). Montes et al. (1996) also reported that in Galicia, Spain, the prevalence of infection in experimentally exposed oysters varied significantly with location. In Ireland, the prevalence, intensity and seasonality of infection was very similar in a stock that had been exposed to B. ostreae for 22 years and a stock infected for 5 years. Although these infected oyster stocks were able to maintain themselves over extended periods of time, the prevalence of B. ostreae will likely remain relatively stable without some intervention to improve resistance levels such as breeding for resistance over a number of years (Flannery et al. 2014a).
The breeding of bonamiosis-resistant flat oysters is reported to have some success (Martin et al. 1993; Boudry et al. 1996; Baud et al. 1997; Naciri-Graven et al. 1998, 1999; Culloty et al. 2001; Lallias et al. 2008). However, there is evidence from DNA microsatellite loci analysis that a population bottleneck has occurred during the selection process in some stocks of bonamiosis-resistant O. edulis. The small effective number of breeders are expected to lead to increasing inbreeding and have important consequences for the future management of at least three selected bonamiosis-resistant populations (Launey et al. 2001). To counteract inbreeding, oyster families were selectively bred to produce progeny with a greater genetic diversity in 1998. These families showed enhanced survival and a lower prevalence of infection compared to control oysters in B. ostreae-infected areas (Lapègue et al. 2003). In Ireland at Rossmore, Cork Harbour, following high losses of stock due to bonamiosis, a selective breeding programme commenced in 1988 to produce B. ostreae-resistant oysters using 3 to 4 yr old survivors as broodstock for controlled spawning in land-based spatting ponds. Results from over 30 years of surveillance indicated that this intervention reduced O. edulis mortalities to negligible numbers during the first 4 yr of growth, the prevalence of B. ostreae infection is now low, and no correlation exists between prevalence of infection and oyster mortalities (Lynch et al. 2014).
A controversial approach to developing bonamiosis-resistance was suggested by Morvan et al. (1994) and Morvan et al. (1997) who determined that B. ostreae and not O. edulis were sensitive to the antimicrobial peptides magainin 1 (originally extracted from the skin of the frog Xenopus laevis) and tachyplesin 1 (extracted from haemocytes of the Japanese horseshoe crab Tachypleus tridentatus), which may provide effective gene sequences that could possibly be used to genetically transform molluscs.
References
Arzul, I. and J.P. Joly. 2011. EURL (European Union Reference Laboratory) for Molluscs Diseases: Bonamia sp.
Arzul, I., B. Chollet, C. Garcia, M. Robert, J.-P. Joly, L. Miossec and F. Berthe. 2005a. Ostrea conchaphila: a natural host of Bonamia ostreae? Journal of Shellfish Research 24: 638-639. (Abstract).
Arzul, I., L. Miossec, E. Blanchet, C. Garcia, J.P. Joly, C. Francois and F. Berthe. 2005b. A long term study of bonamiosis in Quiberon Bay, France. In: 8th International Conference on Shellfish Restoration. (Brest, France).
Arzul, I., L. Miossec, E. Blanchet, C. Garcia, C. Francois and J.P. Joly. 2006. Bonamia ostreae and Ostrea edulis: a stable host-parasite system in France? In: Proceedings of the 11th Symposium of the International Society for Veterinary Epidemiology and Economics (ISVEE), Cairns, Queensland, Australia, 6 - 11 August 2006, Theme 1 - Aquatic animal epidemiology: Crustacean and shellfish disease session, Volume T1-2.4.4: pp. 869-873.
Arzul, I., B. Gagnaire, C. Bond, B. Chollet, B. Morga, S. Ferrand, M. Robert and T. Renault. 2009. Effects of temperature and salinity on the survival of Bonamia ostreae, a parasite infecting flat oysters Ostrea edulis. Diseases of Aquatic Organisms 85: 67–75.
Arzul, I., A. Langlade, B. Chollet, M. Robert, S. Ferrand, E. Omner, S. Lerond, Y. Couraleauy, J.P. Joly, C. François and C. Garcia. 2011. Can the protozoan parasite Bonamia ostreae infect larvae of flat oysters Ostrea edulis? Veterinary Parasitology 179: 69-76.
Audemard, C., R. Carnegie, N. Stokes, E. Burreson and M. Bishop. 2005. Salinity effects on the susceptibility to and persistence of Bonamia ostreae and Bonamia sp. in Crassostrea ariakensis. Journal of Shellfish Research 24: 639. (Abstract).
Bachère, E. and H. Grizel. 1983 (1985). Receptivite de trois populations naturelles d'huîtres plates Ostrea edulis L. au protozoaire Bonamia ostreae (Pichot et al., 1980). (In French with English abstract). Revue des Travaux de l'Institut des Pêches Maritimes. 47: 237-240.
Bachère, E., J.-L. Durand and G. Tigé. 1982a. Bonamia ostreae (Pichot and Coll., 1979) parasite de l'huître plate: comparaison de deux méthodes de diagnostic. (In French with English abstract). International Council for Exploration of the Sea CM. 1982/F:28: 10 pp.
Bachère, E., S. Gagneraud, G. Audic and H. Grizel. 1982b. Mise au point de techniques d'isolement de parasites. Compte rendu d'activité. (In French). Laboratoire de Pathologie, I. S. T. P. M., La Trinite sur Mer. n° 82/2787: 21 pp.
Bachère, E., M. Comps and H. Grizel. 1986. Infections experimentales de l'huître plate Ostrea edulis L. par le protozoaire Bonamia ostreae. In: Vivarès, C. P., Bonami, J.-R., Jaspers, E. (eds.). Pathology in marine aquaculture, Special Publication No. 9., European Aquaculture Society, Bredene, Belgium. pp. 127-132. (In French with English abstract).
Balouet, G. and M. Poder. 1985. A consideration of the cellular reactions in bivalve molluscs, with emphasis on haemocytic diseases. In: Ellis, A.E. (ed.) Fish and Shellfish Pathology. Academic Press, London. pp. 381-385.
Balouet, G., J. Poder and A. Cahour. 1983. Haemocytic parasitosis: morphology and pathology of lesions in the French flat oyster, Ostrea edulis L. Aquaculture 34: 1-14.
Balseiro, P., R.F. Conchas, J. Montes, J. Gómez-León, B. Novoa and A. Figueras. 2006. Comparison of diagnosis techniques for the protozoan parasite Bonamia ostreae in flat oyster Ostrea edulis. Aquaculture 261: 1135-1143.
Baud, J.-P., A. G‚rard and Y. Naciri-Graven. 1997. Comparative growth and mortality of Bonamia ostreae-resistant and wild flat oysters, Ostrea edulis, in an intensive system. I. First year of experiment. Marine Biology 130: 71-79.
Beare, W.E., S.C. Culloty and G. Burnell. 1998. Some observations on spatial and temporal variation in prevalence of infection of Bonamia ostreae (Pichot et al., 1980) in the native flat oyster Ostrea edulis (L.) in Galway Bay, Ireland. Bulletin of the European Association of Fish Pathologists 18: 39-42.
Berthe, F. 2004. Report about mollusc diseaes. Mediterranean aquaculture diagnostic laboratories 49: 33-48.
Bodoy, A., S. Bougrier, P. Geairon, J. Garnier, V. Boulo and S. Heurtebise. 1991. Does the prevalence of Bonamia and Marteilia diseases be reduced on flat oysters (Ostrea edulis) of Atlantic and Mediterranean origin, when they are reared together with the Japanese oyster (Crassostrea gigas) in tidal ponds? International Council for Exploration of the Sea C.M. 1991/K:28: 10 pp.
Bonami, J.R., C.P. Vivarès and M. Brehélin. 1985. Étude d'une nouvelle haplosporidie parasite de l'huître plate Ostrea edulis L.: morphologie and cytologie de différents stades. Protistologica 21: 161-173. (French, English Summary).
Boudry, P., B. Chatain, Y. Naciri-Graven, C. Lemaire and Andrérard. 1996. Genetical improvement of marine fish and shellfish: a French perspective. Proceedings of FOID ‘96 5: 141-150.
Bougrier, S., G. Tigé, E. Bachère and H. Grizel. 1986. Ostrea angasi acclimatization to French coasts. Aquaculture 58: 151-154.
Boulo, V., E. Mialhe, H. Rogier, F. Paolucci and H. Grizel. 1989. Immunodiagnosis of Bonamia ostreae (Ascetospora) infection of Ostrea edulis L. and subcellular identification of epitopes by monoclonal antibodies. Journal of Fish Diseases 12: 257-262.
Brehélin, M., J.R. Bonami, F. Cousserans and C.P. Vivarès. 1982. Existence de formes plasmodiales vraies chez Bonamia ostreae parasite de l'huître plate Ostrea edulis. [True plasmodial forms exist in Bonamia ostreae, a pathogen of the European flat oyster Ostrea edulis.]. Compte Rendu Hebdomadaire des Séances de l'Académie des Séances, Paris. Série III 295: 45-48. (In French with English abstract).
Bucke, D. 1988. Pathology of bonamiasis. Parasitology Today 4: 174-176.
Bucke, D. and S. Feist. 1985. Bonamiasis in the flat oyster, Ostrea edulis, with comments on histological techniques. In: A.E. Ellis (ed.). Fish and Shellfish Pathology, Proceedings of a Symposium, 20-23 September 1983, Plymouth Polytechnic, Plymouth. Academic Press, London. pp. 387-392.
Bucke, D. and B. Hepper. 1987. Bonamia ostreae infecting Ostrea lutaria in the U.K. Bulletin of the European Association of Fish Pathologists 7: 79-80.
Bucke, D., B. Hepper, D. Key and R.C.A. Bannister. 1984. A report on Bonamia ostreae in Ostrea edulis in the UK. International Council for Exploration of the Sea CM 1984/K:9: 7 pp.
Cáceres-Martínez, J., J.A.F. Robledo and A. Figueras. 1995. Presence of Bonamia and its relation to age, growth rates and gonadal development of the flat oyster, Ostrea edulis, in the Ría de Vigo, Galicia (NW Spain). Aquaculture 130: 15-23.
Cao, A., J. Fuentes, P. Comesaña, S.M. Casas and A. Villalba. 2009. A proteomic approach envisaged to analyse the bases of oyster tolerance/resistance to bonamiosis. Aquaculture 295: 149–156.
Carrasco, N., A. Villalba, K.B. Andree, M.Y. Engelsma, B. Lacuesta, A. Ramilo, I. Gairín and M.D. Furones. 2012. Bonamia exitiosa (Haplosporidia) observed infecting the European flat oyster Ostrea edulis cultured on the Spanish Mediterranean coast. Journal of Invertebrate Pathology 110: 307-313.
Carnegie, R. and B. Barber. 1998. Growth, mortality, and Bonamia ostreae prevalence of cultured Ostrea edulis at two sites in the Daramiscotta River, Maine. Abstracts of the First Annual Northeast Aquaculture Conference and Exposition, Rockport, Maine, USA, November 18-19, 1998. pp. 19-20 (Abstract).
Carnegie, R.B. and B.J. Barber. 1999. Impact of Bonamia ostreae on cultured Ostrea edulis at two sites on the Damariscotta River, Maine. Journal of Shellfish Research 18: 296. (Abstract).
Carnegie, R.B. and N. Cochennec-Laureau. 2004. Microcell parasites of oysters: recent insights and future trends. Aquatic Living Resources 17: 519-528.
Carnegie, R.B. and M.Y. Engelsma. 2014. Microcell parasites of molluscs: introduction to DAO Special 7. Diseases of Aquatic Organisms 110: 1-4.
Carnegie, R.B., D.L. Distel and B.J. Barber. 1997. Amplification and sequencing of the Bonamia ostreae 18S rDNA gene: phylogenetic considerations and applications. Journal of Shellfish Research 16: 328. (Abstract).
Carnegie, R.B., B.J. Barber, D.L. Diste and S.C. Culloty. 1999. Development of PCR and in situ hybridization assays for detection of Bonamia ostreae in flat oysters, Ostrea edulis. Journal of Shellfish Research 18: 711-712. (Abstract).
Carnegie, R.B., B.J. Barber, D.L. Distel and S.C. Culloty. 2000a. Development of a PCR assay for detection of Bonamia ostreae in flat oysters, Ostrea edulis. Journal of Shellfish Research 19: 643. (Abstract).
Carnegie, R.B., B.J. Barber, S.C. Culloty, A.J. Figueras and D.L. Distel. 2000b. Development of a PCR assay for detection of the oyster pathogen Bonamia ostreae and support for its inclusion in the Haplosporidia. Diseases of Aquatic Organisms 42: 199-206.
Carnegie, R.B., B.J. Barber and D.L. Distel. 2001. Detection of the flat oyster (Ostrea edulis) parasite Bonamia ostreae by fluorescent in situ hybridization. Journal of Shellfish Research 20: 542. (Abstract).
Carnegie, R.B., B.J. Barber and D.L. Distel. 2003. Detection of the oyster parasite Bonamia ostreae by fluorescent in situ hybridization. Diseases of Aquatic Organisms 55: 247-252.
Chagot, D., D. Hervio, C. Mourton, V. Boulo, E. Mialhe and H. Grizel. 1989. Interactions between Bonamia ostreae (Ascetospora) and hemocytes of the flat oyster (Ostrea edulis) and the cup shaped oyster (Crassostrea gigas): in vitro analysis of entry mechanisms. Developmental and Comparative Immunology 13: 409.
Chagot, D., V. Boulo, D. Hervio, E. Mialhe, E. Bachère, C. Mourton and H. Grizel. 1992. Interactions between Bonamia ostreae (Protozoa: Ascetospora) and hemocytes of Ostrea edulis and Crassostrea gigas (Mollusca: Bivalvia): entry mechanisms. Journal of Invertebrate Pathology 59: 241-249.
Cigarría, J. and R. Elston. 1997. Independent introduction of Bonamia ostreae, a parasite of Ostrea edulis to Spain. Diseases of Aquatic Organisms 29: 157-158.
Cochennec, N., D. Hervio, B. Panatier, V. Boulo, E. Mailhe, H. Rogier, H. Grizel, and F. Paolucci. 1992. A direct monoclonal antibody sandwich immunoassay for detection of Bonamia ostreae (Acetospora) in hemolymph samples of the flat oyster Ostrea edulis (Mollusca: Bivalvia). Diseases of Aquatic Organisms 12: 129-134.
Cochennec, N., T. Renault, P. Boudry, B. Chollet and A. Gerard. 1998. Bonamia -like parasite found in the Suminoe oyster Crassostrea rivularis reared in France. Diseases of Aquatic Organisms 34: 193-197.
Cochennec, N., F. LeRoux, F. Berthe and A. Gerard. 2000. Detection of Bonamia ostreae based on small subunit ribosomal probe. Journal of Invertebrate Pathology 76: 26-32.
Cochennec-Laureau, N., M. Auffret, T. Renault and A. Langlade. 2003. Changes in circulating and tissue-infiltrating hemocyte parameters of European flat oysters, Ostrea edulis, naturally infected with Bonamia ostreae. Journal of Invertebrate Pathology 83: 23-30.
Comesaña, P., S.M. Casas, A. Cao, E. Abollo, I. Arzul, B. Morga and A. Villalba. 2012. Comparison of haemocytic parameters among flat oyster Ostrea edulis stocks with different susceptibility to bonamiosis and the Pacific oyster Crassostrea gigas. Journal of Invertebrate Pathology 109: 274-286.
Comps, M. 1983. Culture in vitro de Bonamia ostreae parasite hémocytaire de l'huître plat Ostrea edulis L. [Culture in vitro of Bonamia ostreae hemocytic parasite of the flat oyster Ostrea edulis L.]. Compte Rendu Hebdomadaire des Séances de l'Académie des Séances, Paris. Série III 296: 931-933. (In French, English Abstract).
Comps, M. 1985. Haemocytic disease of the flat oyster. In: Sindermann, C.J. (ed), Fiches d'Identification des Maladies and Parasites des Poissons, Crustacés and Mollusques. Conseil International pour l'Exploration de la Mer, Copenhague, Fiche 18: pp. 1-5.
Comps, M., G. Tigé and H. Grizel. 1980. Étude ultrastructurale d'un protiste parasite de l'huître Ostrea edulis. Comptes Rendus Académie des Sciences Paris, Série D 290: 383-384.
Conchas, R.F., J. Santamarina, A. Lama, M.A. Longa and J. Montes. 2003. Evolution of bonamiosis in Galicia (NW Spain). Bulletin of the European Association of Fish Pathologists 23: 265-272.
Corbeil, S., I. Arzul, B. Diggles, M. Heasman, B. Chollet, F.C.J. Berthe and M.S.J. Crane. 2006. Development of a TaqMan PCR assay for the detection of Bonamia species. Diseases of Aquatic Organisms 71: 75-80.
Cronin, M.A., S.C. Culloty and M.F. Mulchay. 2001. Lysozyme activity and protein concentration in the haemolymph of the flat oyster Ostrea edulis (L.). Fish and Shellfish Immunology 11: 611-622.
Culloty, S.C. and M.F. Mulcahy. 1996. Season-, age-, and sex-related variations in the prevalence of bonamiasis in flat oyster (Ostrea edulis L.) on the south coast of Ireland. Aquaculture 144: 53-63.
Culloty, S.C. and M.F. Mulcahy. 2007. Bonamia ostreae in the native oyster Ostrea edulis. A review. Marine and Environmental Health Series 29: 1-36.
Culloty, S.C., B. Novoa, M. Pernas, M. Longshaw, M.F. Mulcahy, S.W. Feist and A. Figueras. 1999. Susceptibility of a number of bivalve species to the protozoan parasite Bonamia ostreae and their ability to act as a vector for this parasite. Diseases of Aquatic Organisms 37: 73-80.
Culloty, S.C., M.A. Cronin and M.F. Mulcahy. 2001. An investigation onto the relative resistance of Irish flat oysters Ostrea edulis L. to the parasite Bonamia ostreae (Pichot et al. 1980). Aquaculture 199: 229-244.
Culloty, S.C., M.A. Cronin and M.F. Mulcahy. 2003. Possible limitations of diagnostic methods recommended for the detection of the protistan, Bonamia ostreae in the European flat oyster, Ostrea edulis. Bulletin of the European Association of Fish Pathologists 23: 67-71.
Culloty, S.C., M.A. Cronin and M.F. Mulcahy. 2004. Potential resistance of a number of populations of the oyster Ostrea edulis to the parasite Bonamia ostreae. Aquaculture 237: 41-58.
da Silva, P.M. and A. Villalba. 2004. Comparison of light microscopic techniques for the diagnosis of the infection of the European flat oyster Ostrea edulis by the protozoan Bonamia ostreae. Journal of Invertebrate Pathology 85: 97-104.
da Silva, P.M., P. Comesaña, J. Fuentes and A. Villalba. 2008. Variability of haemocyte and haemolymph parameters in European flat oyster Ostrea edulis families obtained from brood stocks of different geographical origins and relation with infection by the protozoan Bonamia ostreae. Fish and Shellfish Immunology 24: 551-563.
Des Clers, S. 1991. Models for a Bonamia ostreae epidemic in a cohort of cultured European flat oystres, Ostrea edulis. Aquaculture 93: 253-262.
Elston, R.A. 1990. Bonamiasis of the Eurpoean flat oyster. p. 17-21. In: R.A. Elston. Mollusc diseases: guide for the shellfish farmer. University of Washington Press, Seattle.
Elston, R.A., C.A. Farley and M.L. Kent. 1986. Occurrence and significance of bonamiasis in European flat oysters Ostrea edulis in North America. Diseases of Aquatic Organisms 2: 49-54.
Elston, R.A., M.L. Kent and M.T. Wilkinson. 1987. Resistance of Ostrea edulis to Bonamia ostreae infection. Aquaculture 64: 237-242.
Engelsma, M. and M. Hine. 2009a. Infection with Bonamia ostreae: disease detection, pathogen identification and typing. In: Hill, B., A. Reese, P. Dixon, B. Oidtmann, R. Paley, E. Peeler, G. Stentiford, D. Stone, K. Way, M. Hine, P. Calistri, C. Ippoliti, A. Di Lorenzo, L. Savini, O. Haenen, M. Engelsma (eds), Epidemiology of different agents causing disease in aquatic animals: scientific review and database development, (European Food Safety Authority (EFSA), Parma, Italy), Annex B, pp. 46-48.
Engelsma, M. and M. Hine. 2009b. Infection with Bonamia ostreae: occurrence and distribution. In: Hill, B., A. Reese, P. Dixon, B. Oidtmann, R. Paley, E. Peeler, G. Stentiford, D. Stone, K. Way, M. Hine, P. Calistri, C. Ippoliti, A. Di Lorenzo, L. Savini, O. Haenen, M. Engelsma (eds), Epidemiology of different agents causing disease in aquatic animals: scientific review and database development, (European Food Safety Authority (EFSA), Parma, Italy), Annex B, 49-54.
Engelsma, M.Y., S. Kerkhoff, I. Roozenburg, O.L.M. Haenen, A. van Gool, W. Sistermans, S. Wijnhoven and H. Hummel. 2010. Epidemiology of Bonamia ostreae infecting European flat oyster Ostrea edulis from Lake Grevelingen, The Netherlands. Marine Ecology Progress Series 409: 131-142.
Engelsma, M.Y., S.C. Culloty, S.A. Lynch, I. Arzul and R.B. Carnegie. 2014. Bonamia parasites: a rapidly changing perspective on a genus of important mollusc pathogens. Diseases of Aquatic Organisms 110: 5-23.
Farley, C.A., P.H. Wolf and R.A. Elston. 1988. A long-term study of “microcell” disease in oysters with a description of a new genus, Mikrocytos (g. n.) and two new species, Mikrocytos mackini (sp. n.) and Mikrocytos roughleyi (sp. n.). Fishery Bulletin 86: 581-593.
Figueras, A.J. 1991. Bonamia status and its effects in cultured flat oysters in the Ria de Vigo, Galicia (N.W. Spain). Aquaculture 93: 225-233.
Figueras, A. and J.A.F. Robledo. 1994. Bonamia ostreae present in flat oysters (Ostrea edulis) does not infect mussels (Mytilus galloprovincialis). Bulletin of the European Association of Fish Pathologists 14: 98-100.
Fisher, W.S. 1988. In vitro binding of parasites (Bonamia ostreae) and latex particles by hemocytes of susceptible and insusceptible oysters. Developmental and Comparative Immunology 12: 43-53.
Flannery, G., S.A. Lynch, J. Carlsson, T.F. Cross and S.C. Culloty. 2014a. Assessment of the impact of a pathogen, Bonamia ostreae, on Ostrea edulis oyster stocks with different histories of exposure to the parasite in Ireland. Aquaculture 432: 243-251.
Flannery, G., S.A. Lynch, M. Longshaw, D. Stone, P. Martin, A. Ramilo, A. Villalba and S.C. Culloty. 2014b. Interlaboratory variability in screening for Bonamia ostreae, a protistan parasite of the European flat oyster Ostrea edulis. Diseases of Aquatic Organisms 110: 93-99.
Friedman, C.S. and F.O. Perkins. 1994. Range extension of Bonamia ostreae to Maine, U.S.A. Journal of Invertebrate Pathology 64: 179-181.
Friedman, C.S., T. McDowell, J.M. Groff, J.T. Hollibaugh, D. Manzer and R.P. Hedrick. 1989. Presence of Bonamia ostreae among populations of the European flat oyster, Ostrea edulis Linn‚, in California, USA. Journal of Shellfish Research 8: 133-137.
Grizel, H. 1983. Impact de Marteilia refringens et de Bonamia ostreae sur l'ostréiculture bretonne. (In French with English abstract). Conseil International pour l'Exploration de la Mer C.M. 1983/Gen:9: 30 pp.
Grizel, H. 1987. Les maladies des mollusques: étiologie and progrès récents des recherches. Oceanis 13: 357-370. (In French with English abstract).
Grizel, H. 1997. Les maladies des mollusques bivalves: risques and prévention. Revue Scientifique and Technique de l'Office International des Epizooties 16: 161-171.
Grizel, H., M. Comps, D. Raguenes, Y. Leborgne, G. Tige and A.G. Martin. 1982. Bilan des essais d'acclimatation d'Ostrea chilensis sur les cotes de Bretagne. Revue des Travaux de l'Institut des Pêches Maritimes. 46: 209-225.
Grizel, H., E. Mialhe, D. Chagot, V. Boulo and E. Bachère. 1988. Bonamiasis: A model study of diseases in marine molluscs. American Fisheries Society Special Publication 18: 1-4.
Hartikainen, H., O.S. Ashford, C. Berney, B. Okamura, S.W. Feist, C. Baker-Austin, G.D. Stentiford and D. Bass. 2014. Lineage-specific molecular probing reveals novel diversity and ecological partitioning of haplosporidians. The International Society for Microbial Ecology Journal (ISME J) 8: 177-186.
Hervio, D., E. Bachère, E. Mialhe and H. Grizel. 1989. Chemiluminescent responses of Ostrea edulis and Crassostrea gigas hemocytes to Bonamia ostreae (Ascetospora). Developmental and Comparative Immunology 13: 449. (Abstract).
Hervio, D., D. Chagot, P. Godin, H. Grizel and E. Mialhe. 1991. Localization and characterization of acid phophatase activity in Bonamia ostreae (Ascetospora), an intrahemocytic protozoan parasite of the flat oyster Ostrea edulis (Bivalvia). Diseases of Aquatic Organisms 12: 67-70.
Hervio, D., E. Bachère, V. Boulo, N. Cochennec, V. Vuillemin, Y. Le Coguic, G. Cailletaux, J. Mazuri‚ and E. Mialhe. 1995. Establishment of an experimental infection protocol for the flat oyster, Ostrea edulis, with the intrahaemocytic protozoan parasite, Bonamia ostreae: application in the selection of parasite-resistant oysters. Aquaculture 132: 183-194.
Hill, B., A. Reese, P. Dixon, B. Oidtmann, R. Paley, E. Peeler, G. Stentiford, D. Stone, K. Way, M. Hine, P. Calistri, C. Ippoliti, A. Di Lorenzo, L. Savini, O. Haenen and M. Engelsma. 2010a. Epidemiology of different agents causing disease in aquatic animals: scientific review and database development (Parma, Italy, European Food Safety Authority (EFSA)), 21 p. Annex E, pp. 109-116.
Hill, K.M., R.B. Carnegie, N. Aloui-Bejaoui, R.E. Gharsalli, D.M. White, N.A. Stokes and E.M. Burreson. 2010b. Observation of a Bonamia sp. infecting the oyster Ostrea stentina in Tunisia, and a consideration of its phylogenetic affinities. Journal of Invertebrate Pathology 103: 179-185.
Hill, K.M., N.A. Stokes, S.C. Webb, P.M. Hine, M.A. Kroeck, J.D. Moore, M.S. Morley, K.S. Reece, E.M. Burreson and R.B. Carnegie. 2014. Phylogenetics of Bonamia parasites based on small subunit and internal transcribed spacer region ribosomal DNA sequence data. Diseases of Aquatic Organisms 110: 33-54.
Hine, P.M., N. Cochennec-Laureau and F.C.J. Berthe. 2001. Bonamia exitiosus n.sp. (Haplosporidia) infecting flat oysters Ostrea chilensis in New Zealand. Diseases of Aquatic Organisms 47: 63-72.
Howard, A.E. 1994. The possibility of long distance transmission of Bonamia by fouling on boat hulls. Bulletin of the European Association of Fish Pathologists 14: 211-212.
Hudson, E.B. and B.J. Hill. 1991. Impact and spread of bonamiasis in the UK. Aquaculture 93: 279-285.
ICES. 2004. Trends in important diseases affecting fish and molluscs in the ICES area 1998-2002. International Council for the Exploration of the Sea, ICES Cooperative Research Report No. 265. Copenhagen, Denmark. 26 pp. (Prepared and edited by the Working Group on Pathology and Diseases of Marine Organisms.
Katkansky, S.C. and D.R. Manzer. 1967. Oyster disease and mortality study. Quarterly reports for the periods January 1 to March 31, 1967 and April 1 to June 30, 1967. MRO Reference numbers 67-8 and 67-20. Department of Fish and Game, Marine Resources Operations Laboratory, U. S. Bureau of Commercial Fisheries, Research Contract No. 14-17-0001-1382.
Katkansky, S.C., W.A. Dahlstrom and R.W. Warner. 1969. Observations on survival and growth of the European flat oyster, Ostrea edulis, in California. California Fish and Game 55: 69-74.
Laing, I., P. Dunn, E.J. Peeler, S.W. Feist and M. Longshaw. 2014. Epidemiology of Bonamia in the UK, 1982 to 2012. Diseases of Aquatic Organisms 110: 101-111.
Lallias, D., I. Arzul, S. Heurtebise, S. Ferrand, B. Chollet, M. Robert, A.R. Beaumont, P. Boudry, B. Morga and S. Lapègue. 2008. Bonamia ostreae-induced mortalities in one-year old European flat oysters Ostrea edulis: experimental infection by cohabitation challenge. Aquatic Living Resources 21: 423–439.
Lallias, D., L. Gomez-Raya, C.S. Haley, I. Arzul, S. Heurtebise, A.R. Beaumont, P. Boudry and S. Lapègue. 2009. Combining two-stage testing and interval mapping strategies to detect QTL for resistance to bonamiosis in the European flat oyster Ostrea edulis. Marine Biotechnology 11: 570–584.
Lama, A. and J. Montes. 1993. Influence of depth of culture in the infection of the European flat oyster (Ostrea edulis L.) by Bonamia ostreae. Bulletin of the European Association of Fish Pathologists 13: 17-20.
Lapègue, S., E. Bédier, E. Goyard, L. Dégremont, J.-P. Baud, A. Gérard, P. Goulletquer and P. Boudry. 2003. Apport d'un programme de génétique à une filière de production aquacole : l'exemple de l'ostréiculture. (In French with English summary). Contribution of a genetic program to aquaculture: the example of the shellfishery. In: Actes de Colloque Styli 2003 Ifremer, IFREMER). 8 pp.
Launey, S., M. Barre, A. Gerard and Y. Naciri-Graven. 2001. Population bottleneck and effective size in Bonamia ostreae -resistant populations of Ostrea edulis as inferred by microsatellite markers. Genetic Research 78: 259-270.
Lauckner, G. 1983. Diseases of Mollusca: Bivalvia. In: Kinne, O. (ed.) Diseases of marine animals. Volume II: Introduction, Bivalvia to Scaphopoda, Vol. 2. Biologische Anstalt Helgoland, Hamburg. pp. 477-961. (For information pertaining to Bonamia ostreae see pages 577-580).
Le Bec, C., J. Mazurie, N. Cochennec and Y. le Coguic. 1991. Influence of Crassostrea gigas mixed with Ostrea edulis on the incidence of Bonamia disease. Aquaculture 93: 263-271.
López-Flores, L., V.N. Suarez-Santiago, D. Longet, D. Saulnier, B. Chollet and I. Arzul. 2007. Characterization of actin genes in Bonamia ostreae and their application to phylogeny of the Haplosporidia. Parasitology 134: 1941-1948.
Lynch, S.A., S. Wylde, D.V. Armitage, M.F. Mulcahy and S.C. Culloty. 2005a. The susceptibility of young, prespawning oysters, Ostrea edulis, to Bonamia ostreae. Journal of Shellfish Research 24: 664. (Abstract).
Lynch, S.A., D.V. Armitage, S. Wylde, M.F. Mulcahy and S.C. Culloty. 2005b. The susceptibility of young, prespawning oysters, Ostrea edulis, to Bonamia ostreae. Journal of Shellfish Research 24: 1019-1025.
Lynch, S.A., D.V. Armitage, S. Wylde, M.F. Mulcahy and S.C. Culloty. 2006. Inventory of benthic macroinvertebrates and zooplankton in several European Bonamia ostreae-endemic areas and their possible role in the life cycle of this parasite. Marine Biology 149: 1477–1487.
Lynch, S.A., D.V. Armitage, J. Coughlan, M.F. Mulcahy and S.C. Culloty. 2007. Investigating the possible role of benthic macroinvertebrates and zooplankton in the life cycle of the haplosporidian Bonamia ostreae. Experimental Parasitology 115: 359-368.
Lynch, S.A., M.F. Mulcahy and S.C. Culloty. 2008. Efficiency of diagnostic techniques for the parasite, Bonamia ostreae, in the flat oyster, Ostrea edulis. Aquaculture 281: 17-21.
Lynch, S.A., E. Abollo, A. Ramilo, A. Cao, S.C. Culloty and A. Villalba. 2010. Observations raise the question if the Pacific oyster, Crassostrea gigas, can act as either a carrier or a reservoir for Bonamia ostreae or Bonamia exitiosa. Parasitology 137: 1515-1526.
Lynch, S.A., G. Flannery, T. Hugh-Jones, D. Hugh-Jones and S.C. Culloty. 2014. Thirty-year history of Irish (Rossmore) Ostrea edulis selectively bred for disease resistance to Bonamia ostreae. Diseases of Aquatic Organisms 110: 113-121.
Martin, A.G., A. Gérard, N. Cochennec and A. Langlade. 1993. Selecting flat oysters, Ostrea edulis, for survival against the parasite Bonamia ostreae: assessment of the resistance of a first selected generation. Special Publication of the European Aquaculture Society 18: 545-554.
Martín-Gómez, L., A. Villalba and E. Abollo. 2012. Identification and expression of immune genes in the flat oyster Ostrea edulis in response to bonamiosis. Gene 492: 81-93.
Marty, G.D., S.M. Bower, K.R. Clarke, G. Meyer, G. Lowe, A.L. Osborn, E.P. Chow, H. Hannah, S. Byrne, K. Sojonky and J.H. Robinson. 2006. Histopathology and a real-time PCR assay for detection of Bonamia ostreae in Ostrea edulis cultured in western Canada. Aquaculture 261: 33-42.
McArdle, J.F., F. McKiernan, H. Foley and D.H. Jones. 1991. The current status of Bonamia disease in Ireland. Aquaculture 93: 273-278.
Meuriot, E. and H. Grizel. 1984. Note sur l'impact économique des maladies de l'huître plate en Bretagne. Rapports Techniques de l'Institut Scientifique and Technique des Pêches Maritimes 12: 1-20. (In French).
Mialhe, E., E. Bachère, D. Chagot and H. Grizel. 1988a. Isolation and purification of the protozoan Bonamia ostreae (Pichot et al. 1980), a parasite affecting the flat oyster Ostrea edulis L. Aquaculture 71: 293-299.
Mialhe, E., V. Boulo, R. Elston, B. Hill, M. Hine, J. Montes, P. van Banning and H. Grizel. 1988b. Serological analysis of Bonamia in Ostrea edulis and Tiostrea lutaria using polyclonal and monoclonal antibodies. Aquatic Living Resources 1: 67-69.
Montes, J. 1990. Development of Bonamia ostreae parasitosis of flat oyster, Ostrea edulis, from Galicia, northwest Spain. In: Perkins, F.O. and T.C. Cheng (eds), Pathology in Marine Science. Academic Press, San Diego, pp. 223-227.
Montes, J. 1991. Lag time for the infestation of flat oyster (Ostrea edulis L.) by Bonamia ostreae in estuaries of Galicia (N.W.Spain). Aquaculture 93: 235-239.
Montes, J. and I. Meléndez. 1987. Données sur la parasitose de Bonamia ostreae chez l'huître plate de Galice, côte nord-ouest de l'Espagne. Aquaculture 67: 195-198. (French, English abstract).
Montes, J., A. Villalba, M.C. Lopez, M.J. Carballal and S.G. Mourelle. 1991. Bonamiasis in native flat oysters (Ostrea edulis L.) from two intertidal beds of the Ortigueira Estuary (Galicia, N.W. Spain) with different histories of oyster culture. Aquaculture 93: 213-224.
Montes, J., M.J. Carballal, M.C. Lopez and S.G. Mourelle. 1992. Incidence of bonamiasis in flat oyster, Ostrea edulis L., cultured in Galicia (N.W. Spain). Aquaculture 107: 189-192.
Montes, J., R. Anadón and C. Azevedo. 1994. A possible life cycle for Bonamia ostreae on the basis of electron microscopy studies. Journal of Invertebrate Pathology 63: 1-6.
Montes, J., M.A. Longa and A. Lama. 1996. Prevalence of Bonamia ostreae in Galicia (NW Spain) during 1994. Bulletin of the European Association of Fish Pathologists 16: 27-29.
Montes, J., B. Ferro-Soto, R.F. Conchas and A. Guerra. 2003. Determining culture strategies in populations of the European flat oyster, Ostrea edulis, affected by bonamiosis. Aquaculture 220: 175-182.
Moore, J.D. 2006. 5.2.7 Bonamiasis of Oysters, In: Executive Committee (eds.) Fish Health Section Blue Book, 2014 Edition, Suggested Procedures for the Detection and Identification of Certain Finfish and Shellfish Pathogens, Section 1 Diagnostic Procedures for Finfish and Shellfish Pathogens, Chapter 5 Diseases of Molluscan Shellfish, American Fisheries Society's Fish Health Section.
Morga, B., I. Arzul, B. Chollet and T. Renault. 2009. Infection with the protozoan parasite Bonamia ostreae modifies in vitro haemocyte activities of flat oyster Ostrea edulis. Fish and Shellfish Immunology 26: 836-842.
Morga, B., I. Arzul, N. Faury and T. Renault. 2010. Identification of genes from flat oyster Ostrea edulis as suitable housekeeping genes for quantitative real time PCR. Fish and Shellfish Immunology 29: 937-945.
Morga, B., I. Arzul, N. Faury, A. Segarra, B. Chollet and T. Renault. 2011. Molecular responses of Ostrea edulis haemocytes to an in vitro infection with Bonamia ostreae. Developmental and Comparative Immunology 35: 323-333.
Morga, B., T. Renault, N. Faury and I. Arzul. 2012. New insights in flat oyster Ostrea edulis resistance against the parasite Bonamia ostreae. Fish and Shellfish Immunology 32: 958-968
Morvan, A., E. Bachère, P.P. da Silva, P. Pimenta and E. Mialhe. 1994. In vitro activity of the antimicrobial peptide magainin 1 against Bonamia ostreae, the intrahemocytic parasite of the flat oyster Ostrea edulis. Molecular Marine Biology and Biotechnology 3: 327-333.
Morvan, A., S. Iwanaga, M. Comps and E. Bachère. 1997. In vitro activity of the limulus antimicrobial peptide tachyplesin 1 on marine bivalve pathogens. Journal of Invertebrate Pathology 69: 177-182.
Mourton, C., V. Boulo, D. Chagot, D. Hervio, E. Bachère, E. Mialhe and H. Grizel. 1992. Interactions between Bonamia ostreae (Protozoa: Ascetospora) and hemocytes of Ostrea edulis and Crassostrea gigas (Mollusca: Bivalvia): in vitro system establishment. Journal of Invertebrate Pathology 59: 235-240.
Naciri-Graven, Y., A.-G. Martin, J.-P. Baud, T. Renault and A. Gérard. 1998. Selecting the flat oyster Ostrea edulis (L.) for survival when infected with the parasite Bonamia ostreae. Journal of Experimental Marine Biology and Ecology 224: 91-107.
Naciri-Graven, Y., J. Haure, A. Gérard and J.-P. Baud. 1999. Comparative growth of Bonamia ostreae resistant and wild flat oyster Ostrea edulis in an intensive system. II. Second year of experiment. Aquaculture 171: 195-208.
Narcisi, V., I. Arzul, D. Cargini, F. Mosca, A. Calzetta, D. Traversa, M. Robert, J.P. Joly, B. Chollet, T. Renault and P.G. Tiscar. 2010. Detection of Bonamia ostreae and B. exitiosa (Haplosporidia) in Ostrea edulis from the Adriatic Sea (Italy). Diseases of Aquatic Organisms 89: 79–85.
O'Neill, G., S.C. Culloty and M.F. Mulcahy. 1998. The effectiveness of two routine diagnostic techniques for the detection of the protozoan parasite, Bonamia ostreae (Pichot et al. 1980). Bulletin of the European Association of Fish Pathologists 18: 117-120.
Pascual, M., A.-G. Martin, E. Zampatti, D. Coatanea, J. Defossez and R. Robert. 1991. Testing of the Argentina oyster, Ostrea puelchana, in several French oyster farming sites. International Council for Exploration of the Sea C.M.1991/K:30: 17 pp.
Peeler, E.J., B.C. Oidtmann, P.J. Midtlyng, L. Miossec and R.E. Gozlan. 2011. Non-native aquatic animals introductions have driven disease emergence in Europe. Biological Invasions 13: 1291-1303.
Pichot, Y., M. Comps, G. Tigé, H. Grizel and M.A. Rabouin. 1980. Recherches sur Bonamia ostreae gen. n., sp. n., parasite nouveau de l'huître plate Ostrea edulis L. Revue des Travaux de l'Institut des Pêches Maritimes. 43: 131-140. (In French).
Poder, M., M. Auffret and G. Balouet. 1983. Etudes pathologiques and epidemiologiques des lesions parasitaires chez Ostrea edulis: Premiers resultats d'un recherche prospective comparative chez les principales especes de mollusques des zones ostreicoles de Bretagne nord. (In French with English abstract). (Pathological and epidemiological studies of parasitic diseases of Ostrea edulis: First results from a retrospective and comparative research of main species of molluscs in oyster farm in North Brittany.). Bases biologiques de l'aquaculture, Montpellier, 12-16 decembre 1983, IFREMER. Actes de Colloques 1: 125-138.
Prado-Alvarez, M., B. Chollet, Y. Couraleau, B. Morga and I. Arzul. 2013. Heat shock protein 90 of Bonamia ostreae: characterization and possible correlation with infection of the flat oyster, Ostrea edulis. Journal of Eukaryotic Microbiology 60: 257-266.
Ramilo, A., J.I. Navas, A. Villalba and E. Abollo. 2013. Species-specific diagnostic assays for Bonamia ostreae and B. exitiosa in European flat oyster Ostrea edulis: conventional, real-time and multiplex PCR. Diseases of Aquatic Organisms 104: 149-161.
Ramilo, A., M. González, M.J. Carballal, S. Darriba, E. Abollo and A. Villalba. 2014. Oyster parasites Bonamia ostreae and B. exitiosa co-occur in Galicia (NW Spain): spatial distribution and infection dynamics. Diseases of Aquatic Organisms 110: 123-133.
Reece, K.S., M.E. Siddall, N.A. Stokes and E.M. Burreson. 2004. Molecular phylogeny of the haplosporidia based on two independent gene sequences. The Journal of Parasitology 90: 1111-1122.
Renault, T. 2008. Genomics and mollusc pathogens: trends and perspective. Journal of Veterinary Clinical Science 1: 4-14.
Renault, T., N. Cochennec and H. Grizel. 1995. Bonamia ostreae, parasite of the European flat oyster, Ostrea edulis, does not experimentally infect the Japanese oyster, Crassostrea gigas. Bulletin of the European Association of Fish Pathologists 15: 78-80.
Robert, R., M. Borel, Y. Pichot and G. Trut. 1991. Growth and mortality of the European oyster Ostrea edulis in the Bay of Arcachon (France). Aquatic Living Resources 4: 265-274.
Robert, M., C. Garcia, B. Chollet, I. Lopez-Flores, S. Ferrand, C. Francois, J.P. Joly and I. Arzul. 2009. Molecular detection and quantification of the protozoan Bonamia ostreae in the flat oyster, Ostrea edulis. Molecular and Cellular Probes 23: 264-271.
Rogan, E., S.C. Culloty, T.F. Cross and M.F. Mulcahy. 1991. The detection of Bonamia ostreae (Pichot et al. 1980) in frozen oysters (Ostrea edulis L.) and the effect on the parasite condition. Aquaculture 97: 311-315.
Rogier, H., D. Hervio, V. Boulo, C. Clavies, E. Hervaud, E. Bachère, E. Mialhe, H. Grizel, B. Pau and F. Paolucci. 1991. Monoclonal antibodies against Bonamia ostreae (Protozoa: Ascetospora), an intrahaemocytic parasite of flat oyster Ostrea edulis (Mollusca: Bivalvia). Diseases of Aquatic Organisms 11: 135-142.
Saulnier, D., Y. Reynaud, I. Arzul, L. Miossec, F. Le Roux and C. Goarant. 2007. Emergence de maladies chez les organismes d'intérêt aquacole : quelques scénarios illustrés d'exemples. INRA Productions Animales 20: 207-212.
Thebault, A., N. Cochennec, I. Arzul and T. Renault. 2003. Establishing causal link between an infectious agent and mortalities in marine molluscan aquaculture on the example of Bonamia ostreae and Herpèsvirosis in oysters: proposal of a causal grid analysis. In: 10th Symposium of the International Society for Veterinary Epidemiology and Economics, Vina del Mar, Chile, November 2003, pp. 137-140.
Tigé, G. and H. Grizel. 1982 (1984). Essai de contamination d'Ostrea edulis Linné par Bonamia ostreae (Pichot et al., 1979) en rivière de Crach (Morbinhan). (In French with English abstract). Revue des Travaux de l'Institut des Pêches Maritimes. 46: 307-314.
Tigé, G., H. Grizel, A.G. Martin, A. Langlade and M.A. Rabouin. 1981. Situation épidémiologique consécutive à la présence du parasite Bonamia Ostreae en Bretagne - evolution au cours de l'année 1980. (In French). Science and Pêche, Bulletin Institute Pêches Maritimes 315: 13-20.
Tigé, G., H. Grizel, M.A. Rabouin, N. Cochonnec, G. Audic and A. Langlade. 1982. Maladie hémogtaire de l'huître plate causée par Bonamia ostreae : évolution de la situation épizootiologique en Bretagne au cours de l'année 1981. (In French). Science and Pêche, Bulletin Institute Pêches Maritimes 328: 3-13.
Tigé, G., H. Grizel, N. Cochennec and M.A. Rabouin. 1984. Evolution de la situation épizootiologique en Bretagne en 1983 suite au développement de Bonamia ostreae. (In French with English abstract) Conseil International pour l'Exploration de la Mer C.M. 1984/F:14: 14 pp.
Van Banning, P. 1985. Control of Bonamia in Dutch oyster culture. In: Ellis, A.E. (ed), Fish and Shellfish Pathology. Academic Press, London, pp. 393-396.
Van Banning, P. 1987. Further results of the Bonamia ostreae challenge tests in Dutch oyster culture. Aquaculture 67: 191-194.
Van Banning, P. 1988. Management strategies to control diseases in the Dutch culture of edible oysters. American Fisheries Society Special Publication 18: 243-245.
Van Banning, P. 1990. The life cycle of the oyster pathogen Bonamia ostreae with a presumptive phase in the ovarian tissue of the European flat oyster, Ostrea edulis. Aquaculture 84: 189-192.
Van Banning, P. 1991. Observations on bonamiasis in the stock of the European flat oyster, Ostrea edulis, in the Netherlands, with special reference to the recent developments in Lake Grevelingen. Aquaculture 93: 205-211.
Xue, Q. and T. Renault. 2000. Enzymatic activities in European flat oyster, Ostrea edulis, and Pacific oyster, Crassostrea gigas, hemolymph. Journal of Invertebrate Pathology 76: 155-163.
Zabaleta, A.I. and B.J. Barber. 1996. Prevalence, intensity and detection of Bonamia ostreae in Ostrea edulis L. in the Damariscotta River area, Maine. Journal of Shellfish Research 15: 395-400.
Citation Information
Bower, S.M. (2015): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Bonamia ostreae of Oysters
Date last revised: January 2015
Comments to Susan Bower
- Date modified: