Canadian Aquaculture R&D Review 2015
Table of Contents
Sea Lice
VARIATION IN SEA LICE SETTLEMENT WITHIN AND BETWEEN FAMILIES OF COMMERCIALLY REARED SAINT JOHN RIVER STOCK ATLANTIC SALMON
Decreasing the settlement of sea lice on Atlantic Salmon using natural resistance will benefit the industry by increasing fish welfare, growth, and survival while decreasing the use of therapeutants and the number of downgrades at harvest.
The Atlantic Salmon aquaculture industry is seeking strategies to manage sea lice infestations throughout its marine operations. Chemical therapeutants are often used to remove sea lice from infected fish. However, non-chemical approaches are highly desired by the industry if they prove to effectively reduce the total farm load of sea lice. Exploiting natural resistance of Atlantic Salmon individuals and families to sea lice infection holds promise as a non-chemical means to reduce sea lice loads within commercial aquaculture settings. The Huntsman Marine Science Centre has used its established sea lice infection model to explore the natural variability of 2,227 individual Saint John River Atlantic Salmon to Bay of Fundy sea lice infection in challenges involving 132 families (67 sires and 77 dams) from two year classes. A high degree of variability to sea lice infection resistance is evident within and between families. The heritability of this trait was estimated to be 0.20. Results from these challenges will be used to determine the feasibility of integrating sea lice resistance into the selection scheme of a commercial Atlantic Salmon broodstock program.
Oct. 2010–Sep. 2015
Funded By: Atlantic Canada Opportunities Agency – Atlantic Innovation Fund (ACOA – AIF) Co-Funded By: New Brunswick Innovation Foundation; Northern Harvest Sea Farms; Huntsman Marine Science Centre
Project Lead: Amber Garber (Huntsman Marine Science Centre)
Project Team: Susan Hodkinson, Chris Bridger (Huntsman Marine Science Centre); Jane Tosh (U Guelph); Aaron Craig, Robin Muzzerall (Northern Harvest Sea Farms)
Contact: agarber@huntsmanmarine.ca
PREDICTING TRANSMISSION OF SEA LICE BETWEEN AQUACULTURE SITES IN THE EAST COAST OF CANADA
The objective of this study is to incorporate hydrological data into a statistical disease model to better predict farm-level infections and transmission patterns of sea lice on the east coast of Canada. Our work will help the industry establish a protocol for effective bay-management strategies thus, minimizing the effect on the environment while reducing production costs.
Infectious diseases of marine finfish can have a significant economic impact on the global aquaculture industry. Effective control measures against transmission of pathogens between marine farms are essential to reducing losses of fish from infectious diseases, production costs, and environmental pollution from therapeutic treatments. Farm-to-farm spread of pathogens in Atlantic Salmon aquaculture has been described at different distances in several countries, including Canada. However, we currently lack the understanding of the impact of coastal water movements (hydrology) on the transmission of pathogens. Increasing our understanding of transmission patterns in coastal waters will improve existing aquaculture management areas to allow for more effective coordinated treatment strategies in controlling for specific pathogens.
This project will incorporate both hydrological information and ongoing fish disease surveillance data to develop and validate statistical models for disease transmission patterns of sea lice in coastal waters on the east coast of Canada.
We hypothesize that hydrodynamic variation in space and time is a key driver of farm-level infections and transmission patterns. To explore this hypothesis we will focus on a parasite (sea louse) that continues to cause problems in the salmon aquaculture industry in southwestern New Brunswick.
May 2014–Apr. 2015
Funded By: Canada Excellence Research Chair (CERC) – Aquatic Epidemiology, UPEI
Project Lead: Raphael Vanderstichel (UPEI)
Project Team: Fred Page (DFO); Erin Rees, Larry Hammell, Crawford Revie, Sophie St. Hilaire (UPEI)
Contact: rvanderstich@upei.ca
DEVELOPING A NON-CHEMICAL MEANS TO EFFECTIVELY REMOVE ALL FORMS OF SEA LICE FROM AQUACULTURE SALMON USING WARM WATER
Results of the project are expected to provide the required information for ongoing modification of the commercial sea lice warm water shower device, as well as inform sea lice management strategies.
The sea louse (Lepeophtheirus salmonis) is a globally acknowledged challenge for salmon farming operations and a considerable amount of resources are being expended to manage this pest. Chemo-therapeutants and animal husbandry practices have been traditionally used to keep these parasites under control, but there are now signs that sea lice are becoming resistant to many of the chemicals that are being used and recent studies have shown that some of these chemicals are lethal to non-target organisms. Consequently, many non-chemical alternative treatments for sea lice controls are being tested such as predators (cleaner-fish), traps (either physical or biological), and physical exclusion devices (nets, electrical fields). One of the more promising techniques being developed to remove sea lice from captive salmon is the use of warm water. Recent Canadian innovations have developed a warm water shower which appears to remove all attached stages of sea lice and also prevents the detached sea lice individuals from being returned to the ocean. This project aims to develop protocols for the best application of the warm water shower technique to safely and effectively remove sea lice from Atlantic Salmon, including an understanding of the mechanism involved in sea lice removal using warm water.
Apr. 2014–Mar. 2016
Funded By: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP) Co-Funded By: Kelly Cove Salmon Ltd.
Project Lead: Shawn Robinson (DFO)
Project Team: Steve Neil (DFO); Keng Pee Ang (Kelly Cove Salmon Ltd.)
Collaborators: Kelly Cove Salmon Ltd.
Contact: Shawn.Robinson@dfo-mpo.gc.ca
www.dfo-mpo.gc.ca/science/enviro/aquaculture/acrdp-pcrda/index-eng.htm
FIELD TESTING “GREEN TECHNOLOGY” SEA LICE TRAPS AND DOCUMENTING ON-SITE DYNAMICS OF SEA LICE EARLY LIFE HISTORY
The information gathered during this study on larval distribution and physical abilities indicated that there seems to be some significant interactions on site that may be retaining larvae. Further development is required for the light traps to be effective.
The sea louse, Lepeophtheirus salmonis, continues to be a global problem for salmon farming operations and studies have indicated that sea lice are starting to become resistant to therapeutants with continued exposure, thus there is a need for new approaches. There is also concern that operational practices at farm sites could be contributing to the magnification of sea lice infections on the salmon if control measures are not effective for all sea lice life stages (e.g., eggs, larvae). This field project tested the concept that physical light-based traps, in conjunction with an understanding of the on-site sea lice larval dynamics, can help to control sea lice populations. However, the traps were not successful in reducing sea lice densities within the salmon cages due to confounding effects of ongoing sea lice treatments on the site and a malfunction in the self-cleaning filters. The traps worked very well to continually monitor the larval levels and showed seasonal patterns of abundance. Lab studies revealed that egg strings were capable of successfully hatching without the female being present and resulting larvae were found to be very capable swimmers. Further research should look at combining light traps with another attractant to increase the efficiency of sea lice capture.
Apr. 2012–Mar. 2014
Funded By: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP) Co-Funded By: Kelly Cove Salmon Ltd.
Project Lead: Shawn Robinson (DFO)
Project Team: Frank Powell (Cooke Aquaculture)
Collaborators: Keng Pee Ang (Kelly Cove Salmon Ltd.)
Contact: Shawn.Robinson@dfo-mpo.gc.ca
www.dfo-mpo.gc.ca/science/enviro/aquaculture/acrdp-pcrda/index-eng.htm

Solar powered sea lice light trap attached to a salmon cage in the Bay of Fundy. Photo: Nathaniel Feindel (DFO)
AN INVESTIGATION OF THE RELATIONSHIP BETWEEN ENVIRONMENTAL PARAMETERS, OCEANOGRAPHIC ZONES OF INFLUENCE, AND THE PREVALENCE OF PARASITIC COPEPODS ON THREE-SPINE STICKLEBACK IN BAY D’ESPOIR NEWFOUNDLAND WITH SPECIFIC REFERENCE TO SALMONID AQUACULTURE SITES
The results of this research will help provide information on the potential of wild non-salmonid fish species to act as sea lice reservoirs (with the potential to re-infect farmed fish), as well as a potential predictor of infestation levels in Bay Management Areas.
Salmonid farming in Newfoundland and Labrador (NL) has expanded rapidly over the past decade. Concurrently, the occurrences of sea lice infestation on farmed salmon are increasing in some bays (e.g., Bay D’Espoir and Fortune Bay). It has been suggested that non-salmonid species could act as sea lice reservoirs for future infections and/or could act as predictors for infection rates in wild and farmed fish in subsequent years. Despite this, very little information is available on the interaction of wild, non-salmonid fish species at aquaculture sites and sea lice outbreaks at these sites. Based on the results of a 2013 pilot study in Bay D’Espoir, NL, Three-Spine Stickleback were found to be the most prevalent wild fish species around aquaculture sites, and gill lice were the most abundant species of sea lice observed. Gill lice appear to be capable of completing their life cycle on Three-Spine Stickleback; however, the extent of its impact on farmed salmonids in NL has not been characterized. This project will investigate the potential correlation between the distribution of gill lice and other parasitic sea lice on Three-Spine Stickleback and farmed salmonids in the Bay D’Espoir region.
Apr. 2014–Mar. 2016
Funded By: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP) Co-Funded By: Cold Ocean Salmon Inc.
Project Lead: Harry Murray (DFO)
Project Team: Andry Ratsimandresy, Alexandra Eaves, Sebastien Donnet, Dwight Drover, Sharon Kenny (DFO); Keng Pee Ang (Cold Ocean Salmon Inc.)
Collaborators: Cold Ocean Salmon Inc.
Contact: Harry.Murray@dfo-mpo.gc.ca
www.dfo-mpo.gc.ca/aquaculture/acrdp-pcrda/index-eng.htm
ASSESSING SENSITIVITY TO EMAMECTIN BENZOATE (SLICE®) IN SEA LICE LEPEOPHTHEIRUS SALMONIS FROM FARMED ATLANTIC SALMON IN BRITISH COLUMBIA
As this research is the first effort to document sub-lethal effects of emamectin benzoate (SLICE®), a commonly used sea lice treatment. The results of this project will contribute to both increased knowledge and improved disease management strategies to help minimize the impacts of pathogens on farmed salmon.
Infestation with sea lice (Lepeophtheirus salmonis) is a significant economic burden to commercial salmon aquaculture. While there are a range of sea lice control strategies, in-feed emamectin benzoate (known commercially as SLICE®) is the treatment of choice for sea lice on farmed Atlantic Salmon because of its high efficacy and ease of application. However, recent treatment failures have been linked to resistance to SLICE® within sea lice populations. While in vitro data support the conclusion that sea lice in British Columbia remain sensitive to SLICE®, treatment efficacy is variable among sites. Sublethal effects of SLICE® are poorly documented and if this treatment is to remain an effective management strategy, it is important to determine its effects on treatment survivors and on sea lice prior to mortality. This project will attempt to forward that knowledge By: (1) assessing the hatch rate, developmental rate, and viability of cultured larval sea lice (L. salmonis); (2) generating first generation (F1 generation) sea lice to permit comparative assessments of SLICE® sensitivity with parental sea lice; and (3) performing biological assessments to determine the SLICE® sensitivity of parental and F1 generation sea lice, pre- and post-treatment, from each of three salmon production sites.
Apr. 2014–Mar. 2015
Funded By: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP) Co-Funded By: Marine Harvest Canada Inc.
Project Lead: Simon Jones (DFO)
Project Team: Amelia Mahoney (DFO); Brad Boyce, Diane Morrison (Marine Harvest Canada Inc.)
Collaborators: Marine Harvest Canada Inc.
Contact: Simon.Jones@dfo-mpo.gc.ca
www.dfo-mpo.gc.ca/aquaculture/acrdp-pcrda/index-eng.htm
MONITORING AND MODELLING OF SEA LICE INTERACTION WITH WILD AND FARMED SALMON IN THE BROUGHTON ARCHIPELAGO
This project has helped to improve our understanding of the interactions of sea lice with wild and farmed fish. The results of this research will help inform decisions on the siting and management of finfish aquaculture sites in BC and support the long term health of wild fish populations and the fish farm industry.
The interaction of sea lice with farmed salmon and wild salmon has been the focus of international concern for at least a decade. Health and growth performance issues associated with sea lice infestations continue to be a significant concern for the salmon farming industry globally, driving the implementation of preventative measures in areas where there is the threat of infestation. This project developed a predictive model of the distribution of sea lice originating from fish farms and estimated the number of encounters of out-migrating salmon with sea lice. It also established statistically robust models to capture associations between the sea lice burden on wild fish and conditions on BC fish farms. Modelling was used to associate factors such as year, month, type of seine gear used, fish species, and fish length to the presence of sea lice on wild chum and pink salmon. Using Geographical Information Systems (GIS) software, trajectory maps (maps which show the direction and distance of particle movement) and particle density maps were generated for fish farm locations in the Broughton, as well as corresponding fish farm connectivity tables (tables which describe the overlap of particle exchange between farms).
Apr. 2012–Mar. 2014
Funded By: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP) Co-Funded By: Marine Harvest Canada; Grieg Seafood BC; Mainstream Canada
Project Lead: Peter Chandler (DFO)
Collaborators: Marine Harvest Canada; Grieg Seafood BC; Mainstream Canada
Contact: Peter.Chandler@dfo-mpo.gc.ca
www.dfo-mpo.gc.ca/aquaculture/acrdp-pcrda/index-eng.htm

The Broughton Archipelago area showing the locations of fish farms and the sites sampled during the 2012 monitoring program.
EVALUATION OF THE SEASONAL ABUNDANCE, PREVALENCE AND SPECIES DIVERSITY OF SEA LICE ON NON-SALMONID MARINE FISH SPECIES FROM BAY D’ESPOIR, NEWFOUNDLAND, WITH SPECIFIC REFERENCE TO AREAS NEIGHBOURING ATLANTIC SALMON CAGE SITES
Although the copeopod, Ergasilus labracis, has been listed as present in Newfoundland previously, it has a broad host range and has been reported to be pathogenic to farmed salmonids. Therefore, the potential impact of this parasite on wild and farmed fish populations around Newfoundland should not be underestimated.
This project was designed to evaluate the potential for wild non-salmonids to act as reservoirs for parasitic copepods on the South Coast of Newfoundland (Bay D’Espoir). Due to the expansion of finfish aquaculture on the south coast of Newfoundland in the last two decades, it is imperative to understand the parasite ecology of this region. It is known that many species of marine fish frequent areas around cage sites, including Atlantic Cod, pollock, herring, mackerel and sticklebacks (of at least three different species) including the Three-Spined Stickleback. As such, it is reasonable to hypothesize, based upon observations on the west coast of Canada, that many of these species might also be hosting economically important parasites like Lepeophtheirus spp. and/or Caligus spp. in Newfoundland bays where salmon farming is expanding and infestations have been reported in the past. Observations of L. salmonis on sticklebacks and other non-salmonid species thus may be used as a predictor of infestation levels within Bay D’Espoir and/or neighboring bays. The most common parasite surveyed during the project was Ergasilus labracis (n = 4684). Other parasitic copepods observed on sticklebacks during the survey included chalimus stage Lepeophtheirus spp. (n = 3), adult Argulus alosae (n = 2), and a single Thersitina gasterostei. These observations represent a new host record for E. labracis.
Apr. 2013–Mar. 2014
Funded By: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP) Co-Funded By: Cold Ocean Salmon Inc.
Project Lead: Harry Murray (DFO)
Project Team: Alexandra Eaves, Dwight Drover, Sharon Kenny (DFO)
Collaborators: Keng Pee Ang (Cold Ocean Salmon Inc.)
Contact: Harry.Murray@dfo-mpo.gc.ca
www.dfo-mpo.gc.ca/aquaculture/acrdp-pcrda/index-eng.htm
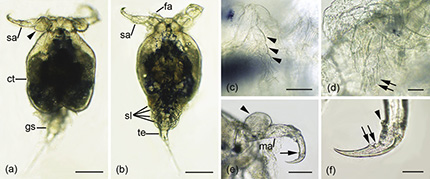
Morphological characteristics of adult female Ergasilus labracis collected from the Three-Spined Stickleback, Gasterosteus aculeatus, in Bay d’Espoir, Newfoundland. Photo: Alexandra Eaves (DFO)
DEVELOPMENT AND PROGRESS OF THE CUNNER BREEDING PROGRAM
The use of a native species of cleaner fish, the Cunner, in Canadian salmonid aquaculture has the potential to significantly decrease the use of chemotherapeutants. The cleaner fish, such as Cunners and Lumpfish, are expected to be part of an Integrated Pest Management Program (IPMP).
Research on greener ways to control sea lice (Lepeophtheirus salmonis), a naturally-occurring ectoparasite, continues to be a priority for Kelly Cove Salmon Ltd. (KCS). Following field trials that showed the efficacy of the Cunner (Tautogolabrus adspersus) in controlling adult sea lice in cages and in the laboratory in 2011, additional Cunners were transferred to a hatchery at the Huntsman Marine Science Centre (HMSC) in St. Andrews in 2012. Significant progress has been made in all aspects of the breeding program since the first successful Cunner spawning in captivity in 2011. A substantial spawn in 2013 resulted in approximately 33,000 healthy juveniles.
With assistance/consultations from Memorial University (D. Boyce and staff) and Scotian Halibut Limited (B. Blanchard and staff), the 2014 spawning season saw improvements in broodstock health, egg collection, and live feed production. This, in addition to new methods of egg collection, resulted in 27 times more eggs collected in 2014 over 2013. From current larval stocks, approximately two to three times more 2014 F1 juveniles are expected. A portion of the 2013 F1 Cunners will be graded and transferred to commercial sea cages in 2015 for field trials on efficacy alongside wild caught Cunners. The remaining Cunners will become broodstock for studies on fecundity and viability of an F2 generation. The overall goal is a fully captive Cunner breeding program at KCS.
May 2012–Dec. 2015
Funded By: NRC – Industrial Research Assistance Program (NRC – IRAP) Co-Funded By: Kelly Cove Salmon Ltd. (KCS)
Project Lead: Keng Pee Ang (KCS)
Project Team: Geoffrey McBriarty, Joshua Francis, Erin Carpenter, Arianna Smith, Jessica Binney, Ashton Bradley (KCS)
Contact: keng.pee.ang@cookeaqua.com

Kelly Cove Salmon Ltd. Cunner. Photo: Roger Wysocki (DFO)
DEVELOPMENT OF BACTERIAL BIOMARKERS OF SALMON MICROBIOTA MEDIATED RESISTANCE AGAINST SEA LOUSE LEPEOPHTHEIRUS SALMONIS
Sea lice, Lepeophtheirus salmonis, are naturally occurring parasites in sea water. Nevertheless, they represent an animal health issue for both wild and farmed salmon and can result in significant economic losses for the salmon aquaculture industry. There are a number of strategies currently being used by farmers to combat sea lice, including the use of chemicals. This has had mixed results and there are concerns about the effects of sea lice treatments on non-target organisms, including lobster.
Numerous research activities have been undertaken to better understand sea lice, their relationship to the marine environment and fish, and the treatments and methods used to reduce their abundance near wild and farmed salmon species. Researchers have been exploring new strategies, such as vaccines and novel drugs for the treatment and removal of sea lice from farmed fish. One innovative approach that is showing some promise is through the use of selective genetic breeding programs to harness the natural resistance to sea lice exhibited by some salmon families.
This study represents the first step in developing sea louse control strategies that combine selective breeding with a probiotic approach; treating farmed salmon with beneficial bacteria isolated from host microbiota that will help protect the salmon against parasites and pathogens. The long term objectives of this research support the development of a sustainable strategy to prevent infections transmitted or triggered via sea lice prevalence and sea lice landing.
Apr. 2013–Mar. 2015
Funded By: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP) Co-Funded By: Kelly Cove Salmon Ltd.; Université Laval
Project Lead: Steven Leadbeater (DFO)
Collaborators: Kelly Cove Salmon Ltd.; Université Laval
Contact: Steven.Leadbeater@dfo-mpo.gc.ca
www.dfo-mpo.gc.ca/aquaculture/acrdp-pcrda/index-eng.htm
THE EFFECTS OF SEA LICE IN MODULATING SALMONID SUSCEPTIBILITY TO VIRUSES
The sea louse, Lepeophtheirus salmonis, is a serious pest of farmed Atlantic Salmon in both eastern and western Canada. As sea lice are ubiquitous in the marine environment and co-occur with endemic viruses, it is inevitable that mixed infections of sea lice and viruses occur on both coasts. Despite their widespread occurrence in both wild fisheries and aquaculture, there have been no controlled studies that explicitly examined the effect of L. salmonis on disease caused by viral pathogens. Infectious hematopoietic necrosis virus (IHNV) is a rhabdovirus that infects wild and cultured salmonid fish throughout the Pacific Northwest of North America. Infectious salmon anemia virus (ISAV) is an orthomyxovirus that infects and causes disease in farmed Atlantic Salmon in eastern Canada. For both viral pathogens, there is a need to better understand if sea lice parasitism is a predisposing factor that influences virus transmission and salmon susceptibility to infection. Our research addresses this issue by integrating parallel investigations into IHNV and ISAV interactions with sea lice in western and eastern Canada, respectively. Our goal is to determine an acceptable level of sea lice infestation at which intervention or pest management strategies may be needed to prevent further damage from viral infection.
This research will guide decision-making regarding the magnitude of sea lice management thresholds for use in salmon aquaculture.
Sep. 2014–Mar. 2016
Funded By: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Lead: Simon Jones (DFO)
Project Team: Kyle Garver (DFO)
Collaborators: Mark Fast (AVC)
Contact: Simon.Jones@dfo-mpo.gc.ca

The salmon louse, Lepeophtheirus salmonis
SEA LICE INFECTION LEVELS ON JUVENILE SALMON DURING EARLY SEAWATER RESIDENCY AND MIGRATION OUT OF THE STRAIT OF GEORGIA
There have been reports that suggest the poor returns of Fraser River Sockeye Salmon could be caused by infections with sea lice acquired from salmon farms during their northern migration from the Strait of Georgia. To determine the potential impact, if any, from salmon farms, background information is needed about the species of sea lice that are present and their numbers on juvenile salmon and non-salmonid hosts. This multi-year project involved sampling of juvenile salmonids and non-salmonids for sea lice during the out migration period. Samples were collected from numerous sites throughout the Strait of Georgia and Johnstone Strait to examine the role that salmon farms may play as a source of sea lice infections on wild fish. Prevalence and abundance of the different sea lice species and their developmental stages were determined. Samples of juvenile Fraser River Sockeye are also examined for the presence of other pathogens and disease. This project provides valuable information on: (1) the species composition and abundance of sea lice in these areas; (2) when and where fish become infected with sea lice; (3) whether patterns of sea lice infection vary among years; and (4) the overall health status of juvenile Fraser River Sockeye Salmon.
Apr. 2010–Mar. 2014
Funded By: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Lead: Stewart Johnson, Richard Beamish, Marc Trudel (DFO)
Project Team: Chrys Neville, Kyle Garver, Simon Jones (DFO)
Contact: Stewart.Johnson@dfo-mpo.gc.ca
DEFINING THE RISK OF SEA LICE INFECTIONS THROUGH THE DEVELOPMENT OF AN UNDERSTANDING OF THE EARLY LIFE HISTORY POPULATION DYNAMICS OF SEA LICE ASSOCIATED WITH ATLANTIC SALMON AQUACULTURE SITES IN THE BAY OF FUNDY
A better understanding of the early life history infection dynamics of sea lice on farms is essential to implementing more effective management measures aimed at disrupting the reproductive cycle of sea lice. Whereas most previous management approaches have considered sea lice larval stages as passive particles to be advected away from farms with oceanographic currents, the data confirming this assumption are scarce. Field sampling has shown that larvae are almost exclusively found in close proximity to active salmon farms, and lab studies have shown that sea lice populations can successfully reproduce in tanks with high flushing rates. These observations suggest that larval stages are far from being passive particles, and that they have certain early life history characteristics that allow them to quickly multiply on salmon farms. The rapid proliferation of sea lice to epidemic levels results in significant impacts to the aquaculture industry, and can have unintended consequences on wild populations, including other fish and invertebrates. This project examines the relative risk of amplification and transmission of infectious outbreaks of sea lice within the salmon aquaculture industry in the Bay of Fundy. The research will provide insight into infection dynamics within a farm as well as an assessment of the risk of transmission of sea lice away from the farm.
Jul. 2014–Mar. 2017
Funded By: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Lead: Shawn Robinson (DFO)
Project Team: Terralynn Lander, Emily Nelson, Fred Page (DFO)
Collaborators: Keng Pee Ang (Cooke Aquaculture Inc.); Gregor Reid (UNB)
Contact: Shawn.Robinson@dfo-mpo.gc.ca
TRANSPORT AND DISPERSAL OF DISCHARGED SEA LICE CHEMICAL THERAPEUTANTS IN SOUTHWEST NEW BRUNSWICK
The use of therapeutant chemicals for treating sea lice outbreaks at salmon farms in southwest New Brunswick has raised concerns about the impacts of such chemicals on the marine environment, and in particular, on non-target organisms. This study used a combination of dye and pesticide concentration measurements, time lapse photography, moored current meters, free floating drifters, GPS tracing and tracking of dye patch edges, in situ fluorometry, computer hydrodynamic, and particle tracking modelling to study and quantify the mixing, flushing, transport, and dispersal of dye and pesticide from net pen tarp and skirt as well as well boat bath treatments. Two pesticides, primarily hydrogen peroxide (Paramove®), azamethiphos (Salmosan®) were used, although, some work was also conducted with deltamethrin (Alphamax®). The study was first conducted in southwest New Brunswick and was later extended to include offshore areas and Grand Manan; this work focused on the effect of dye-therapeutant plumes on zooplankton communities. Site-specific differences in physical environmental parameters influenced the depth, the direction and extent of the pesticide transport. Well boat bath treatments were found to pose the least environmental risk since the quantity of pesticide used was smaller than needed for tarp and skirt treatments, and the rate of dilution associated with well boat based effluent discharges was greater than for tarp or skirt discharges. The results of these studies provide additional science-based data to inform environmental risk assessments and the development of an integrated pest management strategy.
Apr. 2010–Mar. 2014
Funded By: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Lead: Fred Page (DFO)
Contact: Fred.Page@dfo-mpo.gc.ca

Dye released at an aquaculture site in New Brunswick as part of a field trial to study the transport and dispersal of sea lice chemical therapeutants. Photo: Fred Page (DFO)
THE EFFECTS OF SINGLE AND REPEAT LEPEOPHTHEIRUS SALMONIS (SEA LICE) INFECTIONS ON THE HEALTH OF JUVENILE PACIFIC SALMON
There is evidence that different species of Pacific salmon differ in their susceptibility to infections with the sea louse, Lepeophtheirus salmonis, under laboratory conditions. For example, Pink and Coho Salmon have been shown to be less susceptible to sea lice infections than Chinook or Chum Salmon. This multi-year project examined the susceptibility and lethal infection level of juvenile Sockeye, Coho, and Chum Salmon to L. salmonis. In addition, the effects of previous exposure to L. salmonis on susceptibility to infection and the physiological and immunological responses were determined for these species. The project provides managers with tools to help evaluate risks to juvenile salmon, particularly Sockeye Salmon, associated with sea lice infections. The results may be useful in establishing criteria for netpen salmon aquaculture related to siting and site-related thresholds such as production limits and stocking densities.
Apr. 2010–Mar. 2014
Funded By: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Lead: Simon Jones, Stewart Johnson (DFO)
Contact: Simon.Jones@dfo-mpo.gc.ca
BIOASSAYS WITH FARM COLLECTED SEA LICE FROM ALL NEW BRUNSWICK AQUACULTURE BAY MANAGEMENT AREAS USING ALL APPROVED SEA LICE TREATMENT OPTIONS
New Brunswick Department of Agriculture, Aquaculture and Fisheries (NBDAAF) is expected to conduct bioassays to determine the efficacy of all approved sea lice treatment options as outlined under both government regulations and the NB Integrated Pest Management Plan. Completing bioassays is essential to confirm efficacy or resistance of the sea lice population within distinct geographic areas (e.g., Aquaculture Bay Management Areas) to a specific treatment compound and concentration (e.g., Salmosan®). Conversely, bioassays allow confirmation that resistance to a product has been reversed or lost (e.g., SLICE®) within a distinct geographical area. The project will result in completion of bioassays using two approved treatment options on sea lice collected from operating sea cage sites within all five of the NB Aquaculture Bay Management Areas (ABMA). The bioassays will be completed following summer and fall sea lice collections to allow seasonal comparison of the efficacy of each treatment option within NB ABMAs. This process will initiate an essential “early warning diagnostic tool” to alert government, Atlantic Salmon producers and pharmaceutical companies of an impending resistance to a registered sea lice treatment option while maintaining New Brunswick capacity to complete sea lice and related bioassay studies.
Early detection of an impending resistance of sea lice to a registered treatment option is essential for implementation of an effective Integrated Pest Management Plan. Timely results will give industry an opportunity to switch to alternate treatments and further advise for the need for registration of additional products from the pharmaceutical therapeutant pipeline.
Jul. 2014–Dec. 2014
Funded By: NB Department of Agriculture, Aquaculture and Fisheries Co-Funded By: Huntsman Marine Science Centre (HMSC)
Project Lead: Chris Bridger (HMSC)
Project Team: Mike Beattie (NBDAAF)
Contact: Chris.Bridger@huntsmanmarine.ca
THE POTENTIAL OF USING NEWFOUNDLAND STOCK CUNNERS TO CONTROL SEA LICE (LEPEOPHTHEIRUS SALMONIS) ON INFECTED ATLANTIC SALMON SMOLTS: TANK TRIALS
Prolonged use of chemical therapeutants (e.g., SLICE®) to control sea lice (Lepeophtheirus salmonis) infestations on farmed Atlantic Salmon has the potential to lead to the development of local sea lice populations that are resistance to the therapeutant. The use of cleaner fish (e.g., Wrasse sp.) to remove sea lice from Atlantic Salmon in cages has been utilized in Europe with some success. The seriousness of developing chemical resistance in Canada, along with the desire of the industry and regulators to move towards a more sustainable integrated pest management approach, has prompted interest in the potential utilization of local fish species as cleaner fish to supplement and reduce reliance on the use of chemical therapeutants. This project tested the success and efficiency of cunners at feeding on sea lice by stocking cunners in tanks with sea lice-carrying salmon smolts. There was a significant decline in the numbers of sea lice in tanks containing cunners versus a control tank containing infected salmon without cunners. Video work confirmed that cunners did actively pick sea lice off of the salmon. Cunners showed an increase in activity with cohabitation time with salmon. Salmon groups with cunners showed an increased level of blood cortisol (an indicator of physiological stress). The group without cunners showed a decrease (non-significant) in cortisol.
This research has shown that in a tank system, Newfoundland cunners will effectively clean sea lice from Atlantic Salmon smolts. The recognized development of sea lice resistance to chemotherapeutics on the East Coast is a serious concern to the aquaculture industry, and the potential utilization of cleaner fish in this region offers new avenues toward sustainability for the industry.
Apr. 2013–Mar. 2014
Funded By: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP) Co-Funded By: Cold Ocean Salmon Inc.
Project Leads: Dounia Hamoutene, Harry Murray (DFO)
Collaborators: Cold Ocean Salmon Inc.
Contact: Dounia.Hamoutene@dfo-mpo.gc.ca, Harry.Murray@dfo-mpo.gc.ca
www.dfo-mpo.gc.ca/aquaculture/acrdp-pcrda/index-eng.htm

Stop frame photo of a cunner picking sea lice from a salmon. Photo: Lynn Lush (DFO)
DISEASE GENOMICS FOR SALMON LOUSE RESISTANCE IN A COMMERCIAL STRAIN OF ATLANTIC SALMON
The development of Atlantic Salmon that are genetically more resistance to sea lice would economically benefit Canada's aquacultural industry, as well as reducing the use of chemotheraputants. The salmon louse, Lepeophtheirus salmonis, is an ectoparasite that negatively impacts the Canadian aquacultural industry, especially Atlantic Salmon (Salmo salar) in New Brunswick. We are looking at the possibility of a genetic improvement program in the Saint John aquaculture strain of Atlantic Salmon for salmon lice resistance using marker assisted selection, as sea lice resistance is a heritable trait.
By subjecting recent smolts to this species of sea lice, we establish the level of resistance each fish has by counting the number of sea lice attached. Then using genotypic data we look for DNA markers called single nucleotide polymorphisms (SNPs) associated with this resistance. Our current methodology expands from our past studies in that we are now genotyping 50,000 SNPs for fish challenged to sea lice, and 220,000 SNPs for potential broodstock, of which 126,000 work well for the North American subspecies. As the parents of the challenged fish are also genotyped, we will be able to input to 126,000 SNPs, allowing us to cover more of the genome compared to our past studies. SNPs associated with a high level of resistance will be implemented into Atlantic Salmon breeding programs, along with SNPs associated with other economically important traits.
Apr. 2014–Mar. 2017
Funded By: Genome Canada Co-Funded By: Cooke Aquaculture Inc; NRC – IRAP; ACOA
Project Lead: Elizabeth Boulding (U Guelph)
Project Team: Melissa Holborn, Larry Schaeffer, Sarah Loker (U Guelph); Keng Pee Ang, Jake Elliott, Frank Powell (Cooke Aquaculture Inc.); Steven Leadbeater (DFO); Brian Glebe (Genome Atlantic)
Contact: boulding@uoguelph.ca
www.uoguelph.ca/ib/people/faculty/boulding.shtml

MSc. student, Melissa Holborn, taking fin clips for genotyping. Photo: Derek Lapp
.jpg)
Sea lice larvae (Lepeophtheirus salmonis) in the nauplii stage. Photo: Emily Nelson (DFO)
- Date modified: