Center of Expertise in Marine Mammalogy: Scientific Research Report 2009-2011
Table of Contents
- Acknowledgements
- Message from the Director of CEMAM
- 1.0 Population Dynamics
- 1.1 Density dependent-density independent growth in marine mammals (Mike Hammill, Don Bowen, Garry Stenson)
- 1.2 Changes in Reproductive Rates of Northwest Atlantic Harp Seals (G.B. Stenson)
- 1.3 Long term changes in grey seal vital rates are linked to a reduction in population growth rate (Don Bowen)
- 1.4 Ringed seal reproduction in Hudson Bay (Steve Ferguson, Magaly Chambellant)
- 1.5 Using genetics to learn about beluga whale groups in Hudson Bay (Mike Hammill, Lianne Postma)
- 2.0 Role of Marine Mammals in the Ecosystem
- 2.1 Summary of the Zonal Advisory Process on the Impacts of Grey Seals on Fish Populations in Eastern Canada (Don Bowen)
- 2.2 At-sea associations in grey seals: insights from a new data-logger (Don Bowen)
- 2.3 Killer whale foraging specialization on Chinook salmon (John K.B. Ford)
- 2.4 Blue whale feeding ecology in the St Lawrence River estuary (Véronique Lesage, Thomas Doniol-Valcroze)
- 2.5 Baleen whale feeding ground oceanography: the oceanographic trap (Yvan Simard)
- 2.6 Arctic killer whale predation (Steve Ferguson)
- 3.0 Marine Mammal - Human Interactions
- 4.0 Relationships with Co-management Boards
- 5.0 Species at Risk
- 6.0 Publications 2009-2011
Acknowledgements
This publication was made possible by the many DFO marine mammal scientists from across Canada providing input and guidance. We acknowledge support staff, reviewers and all those who made valuable contributions toward the production of this publication. In addition, we thank Christine Abraham for her hard work in compiling the report.
Specifically, we would like to thank the contributors who provided the text for this publication:
- Don Bowen - Research Scientist, Halifax, NS
- Thomas Doniol-Valcroze - Biologist, Mont-Joli, QC
- Steve Ferguson - Research Scientist, Winnipeg, MB
- John Ford - Research Scientist, Nanaimo, BC
- Mike Hammill - Research Scientist, Mont-Joli, QC
- Lois Harwood - Biologist, Yellowknife, NWT
- Michel Lebeuf - Biologist, Mont-Joli, QC
- Véronique Lesage - Research Scientist, Mont-Joli, QC
- Lianne Postma - Biologist, Winnipeg, MB
- Peter Ross - Research Scientist, Sidney, BC
- Yvan Simard - Research Scientist, Mont-Joli, QC Becky
- Sjare Research - Scientist, St. John's, NL
- Garry Stenson - Research Scientist, St. John's, NL
Message from the Director of CEMAM
The Centre of Expertise in Marine Mammalogy (CEMAM) of Fisheries and Oceans Canada (DFO) is a virtual centre involving some forty professionals and support staff located in seven laboratories across the country. Research conducted by CEMAM is diverse, ranging from contaminant levels and their effects, population structure and dynamics, feeding ecology, habitat use and requirements, fisheries interactions and environmental impacts of development. Many species of marine mammals in Canada have shown little sign of recovery from over-exploitation in the past, others in the north are still under pressure from harvesting and still others have fully recovered and are not currently experiencing threats.
A key challenge in our science activities is to provide science advice to inform policy and programs for species ranging from those at risk to highly abundance species. The downturn in the world economy has led many governments to review how they deliver programs. This will have effects both on our research and on how we provide advice. At the same time, emerging issues require attention. Climate change although not new is becoming ever more evident. For example, declines in ice cover off the Atlantic will likely limit the southern breeding distribution of harp seals, and possibly favour the northward expansion of others species such as grey seals. This in turn may increase tension with fishers concerning their potential impacts on commercial fisheries. The decline in Arctic ice is expected to lead to increased marine traffic, to provide access to new areas for commercial development. At the same time it will dramatically reduce marine mammal habitat for some species, but may open up new habitat for formerly temperate species to shift northwards. Off Canada's West and East coasts, offshore development, and increased shipping particularly with Asia may also affect marine mammals. Therefore, a better understanding of the role marine mammals play in marine ecosystems, their habitat requirements, and the impacts of noise and cumulative effects of development are but a few of the issues that will need increased research and management advice in coming years.
Advances in technology, the development of multi-disciplinary databases, and more powerful analytical methods have had a major positive impact on marine mammal research. With the development of new sensors, marine mammals can now transmit oceanographic information from remote areas, difficult to sample by ship, to satellites. These data are being used by oceanographers to improve the accuracy of ocean circulation models. The use of miniaturized acoustic transmitters and receivers on marine mammals and their prey are providing new ways to study how marine mammals associate at sea and on predator-prey interactions. Biopsy sampling from large whales can now be used to provide information on stress levels and reproductive status in addition to information on diet, contaminants and genetics. New analyses involving multiple databases have improved our understanding of critical habitat, and the potential for range expansion, e.g. sea otters, to understand more about predator-prey interactions, and to identify ecologically biologically sensitive areas.
The benefits of CEMAM's work can only be fully realized when we ensure it is broadly accessible to others. In addition to the scientific literature, results from our work are available at the Canadian Science Advisory Secretariat (CSAS) website, at our own website, through popular articles, interviews, lectures, and email alerts to new publications.
This report is only a sample of some of the activities undertaken by CEMAM researchers. It synthesizes results over several years. We have tried to provide a cross-section of activities that highlight new approaches, new findings as well as more general descriptions of phenomena that are affecting marine mammal populations within Canada.
Mike Hammill
1.0 Population Dynamics
1.1 Density dependent-density independent growth in marine mammals (Mike Hammill, Don Bowen, Garry Stenson)
Population numbers naturally tend to change over time under the influence of factors such as competition for resources (e.g., food), predation, immigration and emigration, and environmental variability. An issue that is frequently discussed is whether these changes in population numbers are the result of density-independent or density-dependent processes (Fig. 1). Density dependent factors influence the population in proportion to the population size while density independence factors are unrelated to the size of the population. Density-independent population limitation is often due to environmental changes such as a sudden loss in ice, which can be catastrophic for seal species that require ice as a platform for giving birth and raising their young, leading to high mortality of young. Another example is El Niño, where a sudden change in ocean temperature led to large scale food shortages resulting in starvation.

Figure 1. In density-independent growth, resources are not limiting and in theory the population increases continuously, often exponentially, unless some event occurs. In density-dependent growth, rapid growth can occur when the population is very small, but at some point, food or some other resource becomes limited and population growth slows down to stabilize around a level referred to as the environmental carrying capacity.
In the case of density-dependence, some resource, usually food (or the space available for breeding) increasingly becomes limiting: as a result, the amount of food available per animal declines. Below some threshold, this reduction has the effect of reducing body condition, individual growth rate, reproductive performance, and ultimately survival. The effects of density-dependence are not expressed uniformly across the population. Often the first change is an increase in the mortality rates of juveniles, especially during the first year of life, because they not only must learn to find and capture food on their own, they must consume more energy per body size to support metabolic requirements and growth. Reduced survival rates of the young of the year is often associated with a decrease in the per capita birth rate, as adult females struggle to meet their energy requirements for maintaining themselves and for the added energetic costs of pregnancy and nursing. If conditions get severe enough, adult survival also declines in response to food limitation. Although this is the general pattern and suggests a sequence of responses, there is likely to be some overlap among the changes in vital rates for different age and sex classes in the population.
When the number of births in a population equals the number of deaths, population size will stabilize. A population that has stabilized is considered to be at environmental carrying capacity, termed "K". Although there is a tendency to consider this as a constant, in fact, K may vary over time with longer-term environmental variability. For example, a resource increase may improve environmental conditions, resulting in a new higher K and associated population size while lower resources may result in a decrease in K.
Detecting when a population is at or near carrying capacity is difficult because some short-term population fluctuations can be expected as environmental conditions vary from year to year. Also, for the species we deal with, population size is not measured annually and each estimate of population size is measured with some error. Nevertheless, because the numbers of many marine mammal populations were significantly reduced historically by hunting, many of these species have been, or continue to, increase toward K. Harbour seals and gray whales on the west coast of Canada provide an example of populations that appears to be at or close to K, whereas grey seals on Sable Island are showing signs of density-dependent changes in population growth. Unfortunately, for most marine mammal populations we do not have a long time-series of abundance estimates, so it is not possible to determine if a population is near or at K. Furthermore, some populations are still hunted and it is even more of a challenge to determine what K might be in such situations. If there is insufficient survey data to estimate K, then an alternative approach could help to provide insights as to where K might be.
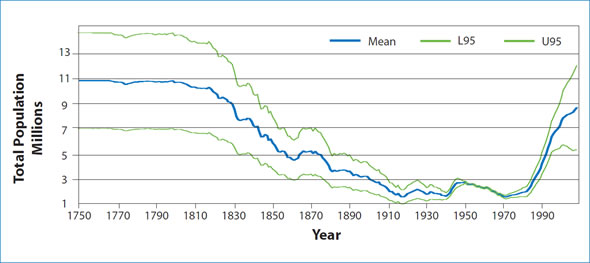
Figure 2: Reconstruction of the Northwest Atlantic harp seal population using information on current population size and data from harvests going back to the late 1700's.
The Northwest Atlantic population of harp seals was significantly reduced due to 300 years of hunting. Since the early 1970s, quota management has allowed the population to increase and during this period we have a relatively long time-series of trend information. The population, which numbered less than 2 million animals in the early 1970s, has grown to around 8.3 million animals in 2008, which is the largest that we have observed in over 6 decades. Over the last 30 years, body condition, growth and age-specific reproductive rates of harp seals have exhibited a declining trend, suggesting that density-dependent factors are affecting the dynamics of this population. However, higher than average pregnancy rates resulted in an estimate of 1.6 million pups born in 2008, the highest in the time series since the 1950s. Such highly variable pregnancy rates make it difficult to determine if the population is approaching carrying capacity. One approach to estimating what K may be is to try to reconstruct the past history of the population to a period prior to when harvesting began. Harp seals have been harvested commercially since the start of the 18th century. In fact the highest harvests occurred during the period of the wooden sealing vessels in the early 1800s. Reconstructing the pre-hunting population using information on how many animals were removed over time is highly uncertain, and in order to assume that this reflects current carrying capacity assumes that ecological conditions that prevailed during the 1800s are similar to environmental conditions today. However, when historical catch data are included in a model and the past size of the population is reconstructed, then the population was estimated to level off at an average of approximately 11 million animals in the early 1800s. Unfortunately, this value is highly uncertain with a possible range of 7 to 15 million animals, but it does provide some indication of the possible maximum size for this population (Fig. 2).
It is important to understand how animals respond to changes in their abundance. Many marine mammal populations were reduced significantly to only a fraction of their pristine population size. Some populations are now recovering and increasing to near-historical numbers. At low populations, there is very little difference between density-independent or density-dependent growth and in some cases, this has led to claims that the populations are 'exploding' and are out of control. At some point, however, populations become limited by food, space or some other factor and density-dependent factors will begin to limit the population, slowing population growth and leading to an eventual levelling off. Understanding the nature of these changes in mortality and reproduction is critical for estimating current abundance and predicting future population growth. The proximity of a population to K can be used as an indication of how healthy it is. A population that is abundant is more likely to withstand sudden perturbations, or catastrophic events, than a small population. Healthy populations are also able to fulfill their traditional ecological role, which for many marine mammals is to stabilize the structure of the marine ecosystem.
1.2 Changes in Reproductive Rates of Northwest Atlantic Harp Seals (G.B. Stenson)
Understanding reproductive rates is critical for understanding the population dynamics of marine mammals. Because it is impossible to survey the entire population, we estimate abundance of harp, hooded, and grey seals by surveying pups and using a population model to scale up these estimates to total population size. Therefore, the reliability of our population estimates are dependent upon accurate annual estimates of age-specific pregnancy rates, particularly as rates vary with changes in population and environmental conditions. However, monitoring such changes is difficult for most species as they require extensive measurements made over long periods. Female reproductive tracts have been collected from harp seals in Newfoundland and southern Labrador waters since the 1950s, with a more systematic program initiated in the 1980s that continues today. Using these data, annual estimates of late-term pregnancy rates, fecundity (proportion of mature females that give birth), and mean age of sexual maturity of Northwest Atlantic harp seals were estimated.
Generally, pregnancy rates for 3-year-old harp seal females were very low (<10 %) with few animals being pregnant. Among the 4- and 5-year-olds, reproductive rates increased during the 1970s, but declined by the mid 1980s to levels similar to, or lower than, those seen in the 1960s. Pregnancy rates for 6-year-olds were low (< 67%) since the mid 1990s when compared with earlier years when rates averaged around 80%. Among the older seals, pregnancy rates were high (80–90%) until the mid 1980s, but then declined. Since then, pregnancy rates fluctuated greatly, but averaged around 60%. Since 2008, pregnancy rates have declined significantly to less than 30%. The proportion of mature females that give birth (referred to as the 'fecundity rate') showed a similar trend, remaining relatively high until the mid 1980s and then declining (Fig. 3). Recent fecundity rates have been highly variable, with years of low pregnancy rates being associated with high levels of late-term abortions.

Figure 3: Annual estimates of the proportion of mature harp seal females that give birth, 1980-2011, based upon the examination of reproductive tracts collected from northeast Newfoundland and southern Labrador, December through February.
The age at which female harp seals became sexually mature also varied over the past 50 years. From 1954 to 1976, the Mean Age of Sexual Maturity (MAM) averaged 5.3 years. Between 1978 and 1987, however, the average age at which female harp seals became sexually mature declined to 4.6 years. By 1990 it had increased by almost a year and throughout the decade, MAM remained fairly constant around 5.6 years. With the exception of 2000, MAM increased during the early 2000s reaching a time-series high of 6.1 years in 2005-06.
Although the direction of the changes we have observed are consistent with a density-dependent response to changes in population size, dramatic changes in the northwest Atlantic ecosystem have also occurred at the same time. A number of physical and biological factors were examined to determine which may have influenced pregnancy rates in harp seals. The overall decline in average fecundity appears to be a response to increases in population size. The interannual variation, however, is best explained by changes in the rates of late-term abortions. In turn, the abortion rates appear to be influenced by early winter ice conditions and food availability, as indicated by capelin abundance in the preceding fall surveys. This suggests that population dynamics of Northwest Atlantic harp seals is being influenced by a complex interplay of internal and external factors that include both physical and biological factors.
1.3 Long term changes in grey seal vital rates are linked to a reduction in population growth rate (Don Bowen)
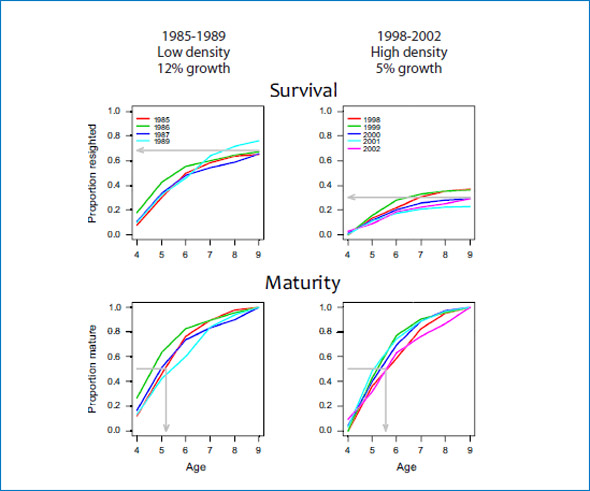
Figure 4: Cumulative proportion of branded female seals resighted on Sable Island between ages 4 and 9 (Survival, first row) and cumulative proportion of resighted females that were seen pregnant or with pup (Maturity, second row) plotted by cohort. The cohorts branded between 1985 and 1989, when the population was at low density and had a high population growth rate are in the first column, and the cohorts branded between 1998 and 2002, when the population density was higher and the growth rate had slowed to 4 to 5% in the second column.
Populations facing resource limitation are expected to exhibit changes in vital rates, such as reduced juvenile survival, delayed maturation and reduced adult survival. Population growth rate of grey seals (Halichoerus grypus) at Sable Island, Nova Scotia has been monitored from 1963 to 2010 by estimating pup production. Recently, the rate of increase in pup production has slowed to 4%/yr from 12%/yr prior to 1997. Periodically between 1969 and 2002, more than 7,000 grey seals were uniquely branded at weaning. Resighting of those branded grey seals, by researchers from the Bedford Institute (D. Bowen, C. den Heyer and J. McMillan) and Dalhousie University (S. Iverson), has been conducted annually from 1983 to 2010 by means of 3-5 weekly censuses of the breeding colony. Resightings of females were used in a mark-recapture analysis to estimate juvenile survival (weaning to age 4), adult survival and age-specific (age 4 to 13) probabilities of first birth. Two groups of cohorts (1985-89 and 1998-2002) were analysed separately to test for temporal changes. Estimated adult survival rates were consistently high (92-99%), but increased slightly during the 1990s. By contrast, the estimated probabilities of first pupping at ages 4 to 13 did not changed and apparent juvenile survival decreased from 78% for the 1980s cohorts to 35% the most recent cohorts (Fig. 4). Although the rate of population growth has slowed significantly, our results show that adult survival has remained high. Therefore, if food limitation is responsible for the observed change in growth rate, then density dependence is being expressed through a marked reduction in juvenile survival.
1.4 Ringed seal reproduction in Hudson Bay (Steve Ferguson, Magaly Chambellant)
Ringed seals are among the smallest and more numerous seal species, and have a northern circumpolar distribution. Sexually mature animals use primarily stable land-fast ice with sufficient snow cover to build sub-nivean birth lairs that are critical for pup survival. As part of a community-based monitoring program established by DFO Winnipeg, ringed seal samples and measurements were collected by Inuit hunters during their fall subsistence harvest in Arviat, Nunavut. Ringed seals were aged by reading growth layer groups in the cementum of decalcified, stained, thin canine tooth sections. Ovaries and testes were frozen upon collection and later examined to assess reproduction.
Growth in length and mass (Fig. 5) of male and female ringed seals were estimated by Gompertz growth curves. There was no sexual dimorphism in length, mass or body condition (fat depth) in western Hudson Bay ringed seals. But, since the females were significantly older, the males may be slightly larger at a given age. Compared with other locations in the Arctic, ringed seals in Hudson Bay were smaller both in length and mass supporting the hypothesis of latitudinal size differences.
In Hudson Bay, females became sexually mature around the age of six years but morphological sexual maturity could be detected as early as three years of age. Male ringed seals seemed to reach sexual maturity around five years of age, but testes mass continued to increase until 10 - 11 years of age. Both sexes appear to mature physically before they reach behavioural sexual maturity. The ages at maturity are in accordance with those of other ringed seals in Hudson Bay and in the rest of the Arctic, albeit at the lower end of the spectrum.
The reproductive cycle of ringed seals in Hudson Bay is similar to other Arctic locations. Pups are born on land-fast or stable pack ice in sub-nivean lairs that require a snow depth on the ground of 20 cm or more to provide sufficient protection against Arctic weather and predators. Nominal birth date for pups is set on the 1st of April but the pupping period could be spread over several weeks. In Hudson Bay, traditional knowledge and recent data converge to an earlier pupping period, starting in February and peaking around mid-March. This supports the hypothesis of a latitudinal gradient of pupping. Pups are weaned before break-up, after nursing for 5 – 7 weeks.
Mating is thought to take place underwater around the time of weaning with a reported peak of male sexual activity from February to April in Hudson Bay. Ringed seal gestation lasts around 10.5 months, with a period of suspended development during the first 2 – 3 months. In late spring, ringed seals undertake their annual moult and require an ice platform to haul-out.
Body condition of ringed seals is poorest in early summer after fasting during the breeding and moulting periods. During the open water period all age-classes are mixed and feed intensively.
When the ice starts to form in late fall, adults gather close to shore to establish territories. During this period, juveniles are actively excluded from these habitats. Adult ringed seals show signs of site fidelity during the winter months and may have a weakly polygynous, resource-defence mating system.
We propose that ringed seal population dynamics follows a decadal cycle that relates to fluctuations in the environment and particularly in the sea ice regime, through changes in ocean productivity and predation pressure. Younger seals in the 2000s grew faster, matured earlier and produced more pups that survived better than those in the 1990s. Such life-history traits are more suggestive of a growing population in the 2000s than in the 1990s. The change in life-history traits during the two study periods supports the hypothesis that ringed seal population dynamics in western Hudson Bay may follow decadal fluctuations of the sea ice regime through atmospheric forcing (e.g., North Atlantic Oscillation). The cycle in ringed seal numbers and reproductive performance is linked to the demographic sensitivity of ringed seal pups to snow cover and ice stability for survival. Thus, a long-term decline of ringed seal fitness in response to current and projected trends in Hudson Bay environmental variables is likely and reinforces the need for long-term data of ice-adapted marine mammals living at the southern limit of their range.
1.5 Using genetics to learn about beluga whale groups in Hudson Bay (Mike Hammill, Lianne Postma)
Across their entire range, beluga whales (Delphinapterus leucas) are known to visit estuaries and river mouths during summer. Some of these estuaries are places of regular aggregations for large numbers of whales and they have traditionally provided good harvesting opportunities for subsistence hunters. This is because the return of belugas is usually predictable and numerous whales are present in a confined environment that facilitates hunting. Indeed, reliable access to these aggregation sites appears to have been important in determining ancient settlement patterns in some Arctic areas.
Several explanations have been proposed to explain this behaviour, but no single reason appears to account for the existence of these aggregations in all cases. The estuaries do appear to be biologically significant and may represent critical habitat for these animals. The fact that belugas continue to return to these locations and persist in occupying them even when subjected to repeated disturbance would appear to underline their importance.
Early researchers have suggested that these summer aggregations of whales consist of distinct groups of animals that should be managed as separate populations or separate management units. The concern is that if any aggregation is harvested too much it may result in abandonment of an estuary. This may not have conservation consequences for the species, but it may result in a loss of biodiversity as well as the loss of food resources for individual villages in specific regions.
To determine if belugas living in and around Hudson Bay are actually distinct groups of whales, we are using DNA markers to compare the genetic profiles of the animals. To help us on this, hunters collect a skin sample from animals they have harvested and return this to us along with information on the date and location that the animal was harvested. We then examine the DNA that we can recover from the skin sample and look for both similarities and differences among the samples collected from different areas in different seasons by different hunters.
Belugas are observed along the eastern Hudson Bay coast, in James Bay and along the western Hudson Bay coast throughout the summer months. The eastern Hudson Bay group is the smallest and likely numbers around 3,000 animals. The belugas that summer along western Hudson Bay number around 57,000 whales and may be the largest gathering of whales in the world. Another 9,000 whales are found in James Bay. The Hudson Bay belugas migrate at the end of summer or in the fall to Hudson Strait where they overwinter, though at least some of the James Bay animals appear to remain in the James Bay area throughout the year. The migration patterns of these whales are very interesting because this is when they appear to mix together and gives them opportunities to mate. Breeding among belugas is thought to occur during the late winter-early spring months. Gestation lasts for 9-12 months, with the calves born the following spring. The calves remain with their mother for 12-18 months. These biological characteristics of beluga whales all contribute to what kinds of patterns we can see when we look at the DNA in the samples we receive from the hunters.
For the genetic analyses, we examine two types of DNA: mitochondrial and nuclear DNA. The mitochondrial DNA (mtDNA) is a type of DNA that is passed on mainly from the mother to her offspring. Looking at information from mtDNA is useful for identifying groups of animals based on their maternal lineages. On the other hand, nuclear DNA (nDNA) is the type of DNA that is more commonly used in forensic science as it is inherited equally from each parent. In belugas, nDNA can therefore be useful for finding out information about breeding patterns i.e. which groups are mating with each other, and what kind of relationships individual whales have to each other.
Among DNA samples collected from beluga whales from the western and eastern coasts of Hudson Bay and from overwintering animals in Hudson Strait, there is little or no differentiation between samples using nuclear DNA. This indicates that animals are interbreeding, probably on the overwintering areas where there are ample opportunities for animals to be together. However, samples from animals that have separated to the summering grounds contained mitochondrial DNA types that were distinct from each other. The strongest mtDNA differences revealed that belugas caught in the summer along the coast of the eastern Hudson Bay (EHB) arc form a genetically distinct group of animals. These EHB whales are genetically most related to the St. Lawrence River belugas, and are quite different from the two main beluga groups in the vicinity, i.e. the western Hudson Bay Stock and the Southeast Baffin Stock.
Using the mtDNA genetic signatures, samples from the northern Quebec (Nunavik) portion of Hudson Strait contain an important proportion (7-31%) of belugas from the EHB stock. This proportion is much higher than would be expected based on the relative size of the western Hudson Bay stock (57,000 animals) compared to the eastern Hudson Bay beluga stock (3,000 animals). This points to differences in the migration patterns between the two groups, particularly when they arrive in the Hudson Strait waters.
Working with colleagues from Laval University, further analyses of the nuclear DNA from the skin samples indicate that parents and offspring, as well as half siblings and other 'close kin', travel in close association with each other both in space and in time. Stronger relationships are shown among mother-daughters than among mother-sons. These results point towards a network of related individuals, particularly among females. These networks move to and from their summering and wintering areas. As such, young belugas are able to learn the specific migratory route of their mother and close relatives, since they will complete 2-4 migrations by the time that they are weaned. This learning of specific migratory routes may have important conservation and management implications. If animals tend to maintain related groups and repeatedly visit the same areas, then loss of these groups would result in the loss of knowledge about the route. Thus, if animals are extirpated from a particular location, then the chances of the area being recolonized are very small. This appears to be the case in some areas around northern Quebec.
These insights provided by genetics are extremely interesting, and they are also supported by information from other types of studies such as local observations, satellite telemetry, stable isotope analyses and comparisons of contaminant signatures. Taken together, the data help to build a foundation of knowledge about beluga whales that can be used to predict how they may be vulnerable to a variety of stressors in their environment. With this in mind, management decisions can be guided to promote conservation of these animals.
2.0 Role of Marine Mammals in the Ecosystem
2.1 Summary of the Zonal Advisory Process on the Impacts of Grey Seals on Fish Populations in Eastern Canada (Don Bowen)
Possible negative impacts of seal predation on fish populations of commercial and conservation interest (e.g. Atlantic cod) continue to be debated. Contributing to this debate is the observed growth in grey seal populations in eastern Canadian waters over the past four decades and the large declines in several fish populations to the point where fishing has been stopped. Natural mortality of adult fish also has been estimated to be unusually high in these collapsed and non-recovering fish populations. Grey seals are hypothesized to have five possible kinds of negative effects on prey populations: 1) predation, 2) competition for food, 3) transmission of parasites causing increased mortality of fish, 4) disruption of spawning causing reduced reproductive success, and 5) other indirect effects on prey productivity.
The Department of Fisheries and Oceans (DFO) held a two-part workshop to review the impacts of seals on Atlantic cod stocks in eastern Canadian waters. The first workshop focused on the nature and quality of available data, and identified data analyses and modeling studies that could be carried out with existing data to more fully address the issue of seal impacts on recovery of commercial fisheries (DFO Proceedings 2008/021). The second workshop reviewed these new analyses (DFO Proceedings 2009/020). However, neither workshop was designed to provide advice in response to question from fisheries managers.
In October 2010, the Department of Fisheries and Oceans convened a 5-day Zonal Advisory meeting of national and international scientists, fishermen, and fisheries managers to provide scientific advice on the following questions: how many grey seals would have to be removed over five years to measurably lower natural mortality on southern Gulf cod and other cod stocks that are experiencing high natural mortality, and what might be the ecosystem responses (e.g. abundance of other predators and prey) to grey seal targeted removal, particularly as it may impact cod recovery?
To attempt to answers these questions participants discussed the result of 32 scientific analyses covering the following topics (DFO Proceedings 2008/021):
- Direct evidence for cod consumption by grey seals;
- Indirect evidence for cod consumption by grey seals;
- Minimum decrease in natural mortality to restore cod populations to reference levels;
- Changes in grey seal abundance, distribution and ecology;
- Grey seals reduction scenarios to restore cod populations;
- Examples of control of large marine predators in other parts of the world; and
- Design of a controlled experiment to test impact of grey seal control on mortality of southern Gulf cod.
In attempting to answer these questions, science is faced with considerable sources of uncertainty. This is because none of the processing affecting seal population dynamics, their consumption of prey, including Atlantic cod, the dynamics of cod and the ecosystem supporting seals, cod, other predators and their prey can be measured without error. Measurement uncertainty can be magnified as scientists attempt to estimate mortality of cod caused by seals because there is uncertainly in estimating the total population of seals, the proportion of the diet containing cod, the size of the cod population, to name just a few.
It is difficult to summarize the results of 5-days of discussion in just a few paragraphs and distilling those analyses and discussions into advice with respect to the management questions was both difficult and accompanied with much debate. Nevertheless, the conclusions of the meeting are summarized in the Science Advisory Report (2010/71). There was broad agreement on a number of conclusions. Grey seals inhabit three linked Atlantic marine ecosystems south of the Laurentian Channel: the southern Gulf of St. Lawrence (NAFO subarea 4T) which freezes in winter causing many fish populations to migrate and overwinter in the warm deeper waters off Cape Breton (NAFO subarea 4Vn) and two Scotian Shelf ecosystems (NAFO subareas 4VsW and 4X). There are distinct cod stocks in each of the three ecosystems. All stocks have shown declines of at least 80% in abundance and all remain low today. Overfishing reduced the stocks in 4T, 4Vn, and 4VsW to low abundance by the early 1990s. Overfishing also contributed to the lesser decline in the 4X stock up to the mid 1990s. Despite the severely reduced fishing mortality, survival of adult cod in 4T has remained at a low level over this period, and the stock has continued to decline. The 4VsW cod stock fell rapidly in the late 1980s leading to collapse, followed by fishery closure in 1993. Stock biomass remained low for over a decade but has recently shown an increase and improved survivorship. The 4X cod stock also experienced high stock mortality and continued to decline after the mid 1990s when fisheries were restricted.
There have been dramatic changes in these ecosystems over the past several decades. Groundfish stocks and fisheries in the southern Gulf have been replaced by small-bodied demersal fishes and invertebrate fisheries. Similarly, the eastern Scotian Shelf ecosystem groundfish fisheries have now been replaced by fisheries on invertebrate species such as shrimp and crab. On the western Scotia Shelf invertebrate fisheries have also increased to unprecedented levels, but some fishing for groundfish continues.
For management purposes, the grey seal population is divided into three herds based upon pupping sites. The largest herd numbering some 260,000 to 320,000 seals, depending on population model assumptions, occurs on Sable Island. The rate of increase of this herd has slowed from 12.8% during the 1980s to approximately 4% in the last 5 years. The southern Gulf of St. Lawrence (Gulf) herd numbers around 55,000-71,000 animals. The coastal Nova Scotia herd is the smallest of the three, numbering around 20,000-22,000 animals. Seals from each of these herds range widely throughout the year while foraging and may contribute to colonization of new breeding sites. Although little is known about historical abundance, current population size is the largest measured in the past several hundred years.
Determining the diet of grey seals relies on indirect methods because there are limited opportunities to directly observe what they eat. The methods used are based on recovery of hard parts such as fish ear bones from stomach contents, intestines, and feces, and the analysis of blubber chemistry in seals and their prey. Each of these methods has strengths and weaknesses. It is also difficult to obtain a representative sample of the diet from grey seals because they range widely and their diet varies by sex, season, area and other factors. Analyses of the above data sources indicate a wide range of values for the percentage of cod in the diet of grey seals; an overall average of 2-7% in 4VsW, and in 4T, from 1% for females in summer to 24% for the only sample of males in winter.
Models of food consumption indicate that grey seal consumption of cod in recent years lies within the range of 4,500 to 20,000 tonnes per year for 4T, and between 3,000 to 11,000 tonnes per year for 4VsW. These estimates themselves have high variance and their wide ranges reflect uncertainty attributable to the assumptions made to address gaps in sampling in 4T and the treatment of the diet data in 4VsW.
Culling is widely practiced as a means to limit predation on livestock and wildlife and can be effective at reducing predator abundance. Culling also has been used to reduce seal species. Although widely practiced, the extent of seal population reduction and the response of targeted prey populations to culls have rarely been evaluated. Results from other predator control programs indicate that unintended consequences in food webs, that will be difficult to predict, are nonetheless commonly observed. Thus, any intervention in the southern Gulf would first require a thorough investigation of the likely multi-species impacts of a cod-seal interaction in this ecosystem, and second would require a carefully designed program that would include clearly-stated objectives and rigorous monitoring of the seal and cod populations and the ecosystem to evaluate the consequences.

Adult male grey seal (brand M720, 24 years old) fitted with a Vemco mobile transceiver (back) and an Argos satellite GPS tag (head).
Photo: W.D. Bowen
Although there was broad agreement on the conclusions above, there was debate concerning the consequences of grey seal consumption of cod on recent and forecasted dynamics of cod, particularly in the 4T area. This is partly because of the added uncertainly assciated with estimating predation mortality on cod rather than just consumption of cod. In 4T, grey seals were considered by some to be significant source of mortality for large cod (>35cm) and other adult, bottom-dwelling fish. Satellite tracking indicates that some grey seals, in particular males, forage where large aggregations of adult cod occur. Digestive tract samples from seals foraging on overwintering aggregations of cod contain a relatively high proportion of cod (about 24% in males and 10% in females, based on intestine samples), and a high proportion (58%) of these cod were greater than 35cm in length. Others were less convinced that available information provided convincing suport for the impact of grey seals on this cod stock.
For 4VsW cod, the magnitude of grey seal predation compared to other sources of mortality varied widely with assumptions of several predation models. Most models left a large portion of mortality unaccounted for and attribute only a small (less than 17%) portion of total cod mortality to seal predation. Comparable information is not available for the mortality inflicted by grey seals on cod in 4X and 4Vn.
Despited the considerable amount and diversity of information brought to bear on this difficult issue, there is still much uncertainty regarding the impact of grey seal predation on the dynamics of cod stocks in Altantic Canada.
2.2 At-sea associations in grey seals: insights from a new data-logger (Don Bowen)

Figure 6: Movements of adult male (orange) and adult female (red) grey seals in the fall of 2009 with the location of at-sea encounters between instrumented seals indicated by white filled circles.
A great deal is known about the behaviour of pinnipeds during the breeding season. By contrast, little is know about the nature and extent of social interactions among pinnipeds while at sea foraging because their behaviour cannot be observed. Investigators at the Bedford Institute of Oceanography and Dalhousie University (Damian C. Lidgard, Ian D. Jonsen, and Sara J. Iverson) are using a novel acoustic technology to examine the nature of spatial and temporal associations among grey seal seals while at sea. Fifteen adult grey seals (Halichoerus grypus) from Sable Island, Canada were fitted with VEMCO Mobile Transceivers (VMTs) and Argos Satellite-GPS transmitters during October 2009. VMTs both transmit the equivalent of an acoustic fingerprint, which identifies each individual seal, and receives these "fingerprints" from other seals also fitted with a VMT. An encounter between two individuals included a cluster of detections that occurred in less than 30 minutes. Argos satellite tags transmitted GPS locations of seals so that the location of interactions could be determined. Two behavioural states (slow and fast movement) were assigned to GPS locations using a state-space model. Tags transmitted for an average of 73 days with an average of 95 locations per day. Twelve of the thirteen VMTs recovered showed seals interacted with other seals while at sea. More that 1,800 detections were recorded in about 200 encounters (Fig. 6). The median duration of an encounter was about 20 minutes and the median number of encounters per seal was 20. The spatial distribution of slow and fast behavioural states indicated that seals exhibited slow movement (thought to represent foraging) while on offshore banks where frequently consumed prey is known to occur, and fast movement (travelling) between these locations. Females were more likely to encounter other tagged seals when engaged in slow movement, while males showed no tendency with respect to either behavioural state. These data (the first of a multi-year study) suggest the occurrence of short-term associations at foraging grounds and provide new insights into the foraging ecology of this marine carnivore. Tagging prey, such as Atlantic cod (Gadus morhua) with coded acoustic tags may also provide a means of investigating predator-prey interactions.
2.3 Killer whale foraging specialization on Chinook salmon (John K.B. Ford)
Killer whales are the oceans' apex predators and one of the most widely distributed mammals in the world. As a species, killer whales can be considered a generalist predator, with a diet that includes a diverse array of different types of prey, including seals, dolphins and large whales, all types of fishes, from small schooling herring to whale sharks, many types of invertebrates, such as squids and octopus, and even reptiles, such as the leatherback turtle. Overall, almost 150 species of marine organisms have been documented as killer whale prey.
Despite this catholic diet of killer whales generally, field studies in several global regions have revealed that local populations of killer whales can have remarkably specialized diets, and may forage selectively for only a very small subset of the prey species that the predator is capable of consuming. These ecologically-specialized populations, or 'ecotypes', may have distinct patterns of seasonal distribution, social structure, behaviour and vocalizations that are strongly influenced by their particular predatory lifestyle. Different ecotypes of killer whales often have overlapping ranges in the same waters, yet they do not mix and are thus reproductively isolated. Such long-term reproductive isolation is thought to have resulted in such genetic divergence among some ecotypes that they are thought to potentially represent distinct species.
Long-term studies of killer whales off Canada's west coast by scientists with DFO's Pacific Biological Station have revealed the existence of three distinct ecotypes in the region, known as 'residents', 'transients', and 'offshores'. Residents feed on a variety of fishes and some squid, but their diet is dominated by salmon. Transients do not appear to feed on any fishes, but instead target marine mammal prey almost exclusively, including seals, sea lions, porpoises, dolphins, and small whales. The diet of offshore killer whales is poorly known but appears to include a high proportion of sharks, which may account for the unusually severe tooth wear seen in this ecotype (the skin of sharks is highly abrasive). These three ecotypes, which are each composed of populations of a few hundred whales, all share coastal waters of British Columbia but they have never been seen to travel together or mix in any way.
Resident killer whales are the best known of the three ecotypes. These whales move seasonally according to the migration patterns of their main prey, Pacific salmon. They've long been known to congregate in good salmon fishing areas along the coast during the summer peak of the salmon migration to spawning rivers, and it was assumed that they likely fed on the five species of Pacific salmon roughly in proportion to each species' availability. However, dedicated field studies on the foraging behaviour of these whales revealed that is was not the case. Using scales and tissue recovered from the water at the site of salmon kills for species identification, it was discovered that resident killer whales feed preferentially on Chinook salmon, one of the rarest of the salmon in the area. Abundant species such as sockeye and pink salmon, which outnumber Chinook by 1000 or more to 1 during their summer migration, are surprisingly not significant in the whales' diet.
The whales' preference for Chinook salmon is understandable – it is by far the largest of Pacific salmon and tends to have the highest oil or fat content, making each fish a higher energy density than any of the other salmonids. Many stocks of Chinook salmon spend their entire life cycle in the whales' coastal habitat, making them available for year round feeding. Why the whales do not prey on the abundant sockeye and pink salmon is more difficult to explain. These salmon species are relatively small in body size and are only available to the whales for a brief time in the summer when transiting coastal waters while en route from the high seas to their spawning rivers. During this period, Chinook salmon are also migrating and are readily available as well. It is likely that the whales have a predatory focus on Chinook salmon throughout the year, and this focus continues even during the summer when other salmonids are more abundant. The whales' foraging tactics are likely finely tuned for efficient predation on Chinook salmon, and the smaller species may be more difficult to catch and are likely not as profitable.
Chinook salmon appear to be so important to resident killer whales that the availability of this single prey species may be critical to their survival. After two decades of slow but steady population growth, the two separate populations of resident killer whales along the west coast, the northern and southern residents, went into a sharp decline in numbers during the late 1990s. Demographic analysis revealed that this decline was driven primarily by a dramatic increase in mortalities during this period, and secondarily by reduced calving rates. Although both the northern and southern populations stabilized and even began increasing slightly in the early 2000s, they were listed Threatened and Endangered, respectively, under Canada's Species at Risk Act in 2003. An analysis of the coast-wide abundance of Chinook salmon over a 25-year period revealed a very strong correlation between whale survival and Chinook salmon abundance. Most striking was that resident killer whale mortalities spiked to levels 2 to 3 times higher than expected during the late 1990s, when Chinook abundance dropped by almost half the long term average for several consecutive years.
As resident killer whale recovery may depend on sufficient availability of Chinook salmon, it is important that we have the best understanding possible of seasonal and spatial patterns of predation by these whales and the potential effects that human fisheries may have on Chinook abundance. Our research is currently focused on improving our understanding of the Chinook salmon stocks that are important to killer whales through genetic stock identification from prey fragments, and estimating the numbers of Chinook salmon that are needed to sustain current whale abundance and provide for future population growth. We are also working closely and collaboratively with whale and salmon scientists and managers both within DFO and with the National Ocean and Atmospheric Administration (NOAA) in the United States to determine whether existing fisheries are potentially having an effect on the recovery of resident killer whales.
2.4 Blue whale feeding ecology in the St Lawrence River estuary (Véronique Lesage, Thomas Doniol-Valcroze)
Feeding is central to an animal's life history and ecology. Large predators do not feed continuously but rather in bouts of intense activity separated by periods of searching, resting or socializing. Moreover, feeding does not occur randomly in space, as animals select precise areas with characteristics of prey density, accessibility and predictability that maximize their chances of meeting their energy requirements. Every summer, blue whales from the endangered North Atlantic population come to the St Lawrence River estuary to feed on dense aggregations of euphausiids. Documenting the timing and location of foraging success is therefore of utmost importance to assess and monitor habitat quality on this feeding ground.
In marine systems, however, feeding happens mostly under the surface and is rarely observable directly. In this study, we have used data-loggers to record, at every second, the depth and swimming speed of 10 blue whales during their dives in the St Lawrence estuary. By detecting the rapid speed changes that are characteristic of lunging behaviour and mouth opening, we have been able to pinpoint the exact moment, depth and location of each feeding attempt. With this information, we have shown that blue whales feed at all times of the diurnal cycle and intensify their feeding activity at night when prey are accessible at shallow depths. This is in contrast to previous assumptions in the literature that blue whales did not feed at night. Using radio-telemetry, we have also been able to describe the habitats where blue whales concentrated their feeding effort, and how different habitats were used at different phases of the tidal cycle (e.g., feeding at the shelf edge when flood tidal currents were concentrating euphausiids against the steep slopes).
Moreover, we have shown that St Lawrence blue whales used optimal strategies to adapt their dive times and feeding effort to the depth of their prey. In particular, feeding rates were consistently higher when blue whales performed short feeding dives at shallow depths. These results suggest that diving predators may judge habitat quality in terms of prey accessibility at shallow depths rather than selecting habitat solely based on prey density or abundance. Taken together, these strategies may allow blue whales to optimize a short seasonal window of feeding opportunity and maximize resource acquisition. Indeed, feeding rates diminished over the summer feeding season, and were negatively correlated with the time each animal spent in a social pair, suggesting a trade-off between feeding and socializing with the approach of the breeding season. Better understanding of the behaviour and feeding ecology of large whales can help predict their responses to environmental changes and anthropogenic pressures.
This project was conducted in collaboration with Robert Michaud and Janie Giard from the Group for Research and Education on Marine Mammals in Tadoussac, Quebec.
2.5 Baleen whale feeding ground oceanography: the oceanographic trap (Yvan Simard)

Figure 7: Example of the functioning of the "oceanographic trap" from the Saguenay–St. Lawrence Marine Park baleen whale feeding ground of the St. Lawrence estuary.

A group of killer whales in the Churchill estuary area taken on the same day predation by killer whales on beluga was observed 20 km to the west in Button Bay, 27 August 2011.
Photo: Pete Ewins
What makes particular ocean areas especially attractive for feeding baleen whales? Several such ecosystem hot spots are "oceanographic traps" for their preferred zooplankton food. An example of how these systems are working is presented in Figure 7, for the baleen whale feeding ground of the Saguenay–St. Lawrence Marine Park.
This traditional feeding ground already existed when the first European whalers arrived 450 years ago. Today, this area is one of the most intensive whale-watching sites of the world. What are the fundamental processes responsible for the making and long persistence of this food-rich area, regularly visited by Northwest Atlantic whales? This is the question a multi-disciplinary team addressed in the ecosystemic and oceanographic research program briefly summarised here.
Using multi-frequency hydroacoustics, oceanographic measurements, plankton sampling, and high-resolution 3D circulation modelling coupled with ground-truthed krill behaviour models, the merged results clearly evidenced how ocean processes combine to trap krill in predictable locations, notably channel and canyon heads, where special habitat properties converge. This functioning mode 1, called "the oceanographic trap", involves here: 1) the underwater topography; 2) the strong and persistent 2-layer estuarine circulation that sorts krill by size and pumps adult krill towards the channel head; 3) the negative phototactism of krill to avoid visual predators, which drives their diel vertical migrations, and which forces them to concentrate under upwelling conditions occurring along slopes every tidal cycle, very strongly and intensively at the head of the Laurentian channel; and finally 4) the advection at semi-diurnal and fortnight tidal frequencies as at lower frequencies, which modulates the whole process by imprinting its variability in the aggregation and dispersion of krill. The krill aggregation process depicted by this research is most likely general and applicable to other whale feeding grounds with this food aggregation mode 1. Details can be found in Simard (2009) and the references listed below.
Present ecosystemic research is addressing the transport coupling between the adult krill source region, in the Gulf, and the aggregation at the channel head in the Marine Park, using a series of ocean observatories that are monitoring the currents and krill biomass at several locations along the transport route and continue over the annual cycle. Eventually, indicators of baleen whale ecosystem state could be developed by merging the observatories results with coupled circulation and krill modelling.
2.6 Arctic killer whale predation (Steve Ferguson)
Killer whales (Orcinus orca) have a global distribution, but many high-latitude populations are not well studied. Anecdotal evidence, sighting reports, Inuit traditional knowledge, and photographic identification indicate that killer whale occurrence in Hudson Bay is increasing. Killer whales were not known to be present in the region prior to the mid-1900s but have since shown an exponential increase in sightings. Killer whales have been observed preying on a number of marine mammal species in Hudson Bay. Of particular concern is predation on bowhead whales (Balaena mysticetus) in Foxe Basin, narwhal (Monodon monoceros) in northwest Hudson Bay, and beluga (Delphinapterus leucas) in southwest Hudson Bay. The impact of killer whale predation on marine mammal species is unknown without long-term studies and direct observation of killer whale hunting behaviour. We conducted a semi-directed interview survey of Traditional Ecological Knowledge to provide additional information on the feeding ecology of killer whales. Local resource users are knowledgeable observers of their environment, and Inuit hunters and community elders have extensive knowledge about killer whales. Using this information and defining killer whale energetic requirements and considering population demography of prey, we can begin to assess the basic requirements of predator–prey dynamics in Hudson Bay marine ecosystem. To estimate predation impact we used a simple mass-balanced marine mammal model that included age structure, population size, and predation rate inputs. For the Hudson Bay region, model results indicated that killer whales do not show strong prey specialization and instead alternatively feed on narwhal and beluga whales early and late in the ice-free season. Evidence does support the conjecture that during the peak of the open water season, killer whale predation can focus on bowhead whale prey. The mixed foraging strategy used by killer whales included seasonal predator specialization and has management and conservation significance since killer whale predation may not be constrained by a regulatory functional response.
3.0 Marine Mammal - Human Interactions
3.1 Harbour seals as indicators of food web contamination in Canada (Peter S. Ross, Michel Lebeuf)
Persistent organic pollutants (POPs) refer to chemicals that possess four key properties: they are persistent, bioaccumulative, toxic, and subject to long-range transport. Members of this class include the polychlorinated biphenyls (PCBs), dioxins (polychlorinated dibenzo-p-dioxins or PCDDs), and DDT. While most POPs have been regulated in Canada and are presently subject to the international Stockholm Convention of 2001, concerns linger owing to the persistence of these contaminants. The use of DDT as an insecticide led to complete reproductive failure of many fish-eating birds in North America and Europe. PCBs have been associated with reproductive failure, immunotoxicity and endocrine disruption in marine mammals. Dioxin-related compounds have been implicated in the failure of lake trout to reproduce in Lake Ontario during the period 1945-80.
Persistent organic pollutants are lipophilic and partition readily into the fatty tissues of biota. The metabolic recalcitrance of POPs results in their biomagnification from one trophic level to the next, with high trophic level species having up to 109 times higher concentrations than surrounding water. Ecotoxicological research and monitoring programs provide insight into conservation priorities for some marine mammal populations, but also into human health risks associated with the consumption of aquatic foods. Since POPs enter the environment from a combination of numerous point and non-point sources, marine mammal studies can provide an integrated signal of contaminants in food webs, and can inform regulations, mitigation, conservation and fisheries management. In addition to local and regional sources, long range transport via atmospheric processes and biological migrations bring contaminants into coastal regions.

Harbour seals haul out on intertidal rocks in the Strait of Georgia, British Columbia, Canada.
Photo: Peter S. Ross.
POPs of concern in Canada include the PCBs, dioxins, furans, organochlorine pesticides including DDT, as well as polybrominated diphenylethers (PBDEs). High levels of PCBs have been reported in British Columbia's resident and transient killer whales (Orcinus orca), and the St Lawrence estuary beluga whales (Delphinapterus leucas). For these Species at Risk Act (SARA)-listed cetaceans, POPs have been identified as conservation-level concerns, requiring scrutiny and action in the context of Recovery Strategies. While the 'legacy' POPs have declined in Canadian marine mammals since regulations were implemented in the 1970s, emerging contaminants include the PBDEs which saw a steady increase in marine mammals from the early 1980s prior to partial source controls in 2004.
Harbour seals (Phoca vitulina) are small pinnipeds that are widely distributed throughout the northern hemisphere. They are found on Canada's Pacific and Atlantic coasts, as well as in the Arctic and the St Lawrence estuary (Fig. 8). They are considered omnivorous but have a preference for small, lipid-rich prey including hake (Merluccius sp.), herring (Clupea sp.), and tomcod (Microgadus sp.). The harbour seal has become a useful 'sentinel' of marine food web contamination because of its abundance, distribution, high trophic level, non-migratory nature, and relative ease of handling.

Figure 8: Harbour seals (Phoca vitulina) are pinnipeds that are widely distributed along Canada's Pacific, Atlantic and Arctic coasts (shaded areas).
In an effort to characterize contaminant levels and risks in this sentinel species, harbour seal pups were live-captured from intertidal haul-out sites using a small craft and manual restraint. Skin/blubber biopsies, as well as blood samples, were collected under aseptic conditions. Samples stored until analysis for PCBs (n = 160) and PBDEs (n = 44) at the DFO Laboratory of Expertise for Aquatic Chemical Analyses (LEACA) in Sidney BC.
Results reveal that moderate concentrations of both PCBs and PBDEs are found in seals from all four study areas (Fig. 9). Despite regulations, PCBs are found at higher concentrations than the PBDEs. PCB levels were highest in seals from the St Lawrence estuary, reflecting their proximity to industrial sources in the region. On the other hand, PBDE concentrations were highest in seals from the Strait of Georgia, British Columbia, likely reflecting the proximity to major municipalities which are know to release PBDEs through sewage effluent (e.g. Vancouver). While the relatively low levels of both PCBs and PBDEs in seals from Newfoundland and Labrador likely indicate minimal local contributions, results nonetheless highlight the ubiquitous distribution of these POPs in coastal food webs.

Figure 9: Harbour seals from four areas in Canada reveal PCB and PBDE contamination of coastal food webs. PCBs continue to be found in high trophic level species, despite regulations enacted in the 1970s. PBDEs face current regulatory scrutiny following rapid increases in biota.
A divergent history of uses, emissions and regulations provide an asynchronous backdrop for PCB and PBDE concentrations in the marine environment, with consequences for adjacent food webs. Harbour seals provide an integrated measure of coastal food web contamination, thereby generating insight into contaminants of concern in the coastal environment. Results provide a backdrop for ecosystem-based management, and help to prioritize chemical regulations, conservation objectives and other management strategies.
3.2 Bowhead Whale Surveys and Satellite Tagging: Beaufort Sea, 2007-2010 (Lois Harwood)
Bowhead whales of the Bering Sea population (also known as the Bering-Chukchi-Beaufort population B-C-B) winter in the Bering Sea, and return annually to summer range in the Canadian Beaufort Sea and Amundsen Gulf. Some of the locations that feeding bowhead whales use in the Canadian Beaufort Sea include areas that have been subject to hydrocarbon exploration activity in the 1980's, and more recently, 2-D ad 3-D seismic surveys in 2001- 2002, 2006-2010. While there was no offshore seismic activity in the Beaufort Sea in 2011, at least two programs are envisaged for 2012. It is also of note that the bowhead whale fall migration route intersects areas of interest to the hydrocarbon industry in the Alaskan Beaufort Sea and in the central Chukchi Sea, including Lease 193 which is active at the present time.
During July, bowheads are widely distributed throughout the offshore Canadian Beaufort Sea, singly or in small (2-3 surfaced animals) groups. By early to mid-August, the whales aggregate to feed in specific, recurrent areas on the summer range. Bowhead feeding aggregations occur where oceanographic conditions favour the concentration of crustaceous zooplankton, their main prey item, and indeed they must seek out areas where prey are adequately concentrated in order to meet their annual energy requirements. Oceanographic features that lead to upwelling of nutrient rich waters are particularly important in determining zooplankton distribution. In the southeastern Beaufort Sea, sampling of plankton in close proximity to feeding bowhead whales revealed predominantly (76-92%) copepods (Limnocalanus macrurus, Calanus hyperboreus, Calanus glacialis), as well as gammariid and hyperiid amphipods, euphausiids, mysids and isopods as the major prey items.
Our bowhead survey and tagging studies were done to update our knowledge of bowhead distribution, habitat use and behaviour in the SE Beaufort Sea, the latter in cooperation with scientists and harvesters from Alaska. Additional outcomes of this work were the provision of real-time bowhead location and sighting data from the aerial survey to other scientists working on a concurrent oceanographic sampling project, and inclusion in a strategy for mitigation of the potential effects of seismic noise on bowheads in the southeast Beaufort Sea.
Strip-transect systematic aerial surveys were conducted over the Canadian Beaufort Sea in late August of 2007, 2008 and 2009 to examine the distribution of bowhead whales (Balaena mysticetus). A total of 24-26 N-S transect lines were flown in each survey, all under favourable survey conditions, along lines of longitude spaced at 15'. A total of 334 bowhead whales (244 sightings) including 10 calves, were observed on-transect by primary observers, mostly as individuals (76.6%) and groups of two (14.3%). The study area was subsequently divided into 20 km x 20 km grid cells, with the grid cell dimensions equal to the transect spacing. Extrapolation of visible, surfaced whale counts on transect segments to unsurveyed areas in each grid cell were summed to yield an estimated 1,320 (95% CI 1036 to 1603) bowheads visible at the surface during the 2007 survey. When an approximate correction for submerged whales was applied, an estimated 5,280 bowheads or at least half of the current estimate of stock size was estimated to have been in the study area at the time of the flying.
Bowheads aggregated in nine geographic locations within the study area in the 2007-2009 survey series. Not all of the nine areas were used in all years, and no more than six areas were used in any given year. The shallow, shelf waters offshore of the Tuktoyaktuk Peninsula were the most attractive to bowheads in all years of the 2007-2009 survey series, with 47.3 % of all whales sighted (66.5% of sightings). The other 8 areas where bowheads aggregated had from 1.5% to 6.3% of the total on-transect bowhead whales, and in total (28.1%) the importance of these areas to bowheads did not equal that of the Tuktoyaktuk Peninsula aggregation area, at least in terms of numbers sighted. The propensity of bowheads to aggregate, and a real-time knowledge of the aggregation areas they use in a given season, provides a framework for the establishment of mitigative measures relating to seismic surveys in the Beaufort Sea.
In 2005, the Alaska Department of Fish and Game began a cooperative research project to study movements and habitat use of the western Arctic stock of bowhead whales (Balaena mysticetus). In 2007-2010, DFO Science and whalers from Canada collaborated on this project to deploy satellite tags on bowhead whales in Canada, to compliment the concurrent deployment of tags in Alaska. In total, 59 satellite transmitters were placed on bowhead whales in Alaska and Canada between 2006 and 2010, 21 of these in Canadian waters. Tagging in consecutive years has allowed us to examine variability in movements, wintering areas, and the timing of migration among years. We have identified several areas of concentrated use throughout the range of bowhead whales, and have documented interactions with seismic activity in the offshore. More detailed analyses are underway, and will include bowhead movements relative to industrial activities and oceanographic factors that influence movements and bowhead foraging.
4.0 Relationships with co-management boards
4.1 Marine Mammal Research in the North: Working with Hunters and Communities (Becky Sjare, Lois Harwood, Steve Ferguson, Veronique Lesage)
Background on Co-management
Co-management approaches are the basis of marine mammal and fish resource conservation throughout much of Canada's northern regions. In general terms, co-management is a process that is rooted in legislation, ensuring local hunters and fishers, other community members, government agencies and various public boards share management and conservation responsibilities for marine mammal and fish resources. Some key features of co-management include shared research project design, implementation and participation at the community level, shared decision-making powers, and the use of traditional ecological knowledge along with the results of scientific research. Integrating these features ensures that research programs are effective and meaningful for all involved and that they address the longer-term management and conservation needs of the species in question.
Most of the co-management bodies in northern regions were established as a requirement of the Inuvialuit, Nunavik, Nunavut and Nunatsiavut Land Claims Agreements. Presently, there are several boards and committees functioning in Canada as a result of these and other land Claims, but the ones most relavent from the persepective of Departmental marine mammal research are the following: 1) the Fisheries Joint Management Committee (FJMC) operating in the Mackenzie Delta/Beaufort Sea since 1986; 2) the Nunavut Wildlife Management Board (NWB) established for the Eastern Arctic region in 1993; 3) the Torngate Joint Fisheries Board (TJFB) created for northern Labrador in 2005; and 4) the Nunavik Marine Region Wildlife Board (NMRWB) established in 2009 for northern Quebec.
The co-management bodies are the main instruments of marine mammal (and fisheries) management within the various land Claims settlement areas. They also provide direction and a framework for the development of marine mammal research partnerships and collaborations with DFO, as well as partial or total funding for the work. Although the governance structure and decision making powers of these bodies does vary, generally each has appointees nominated by Aboriginal Governments and/or organizations, communities, the Minister of Fisheries and Oceans, other federal government departments/agencies and, in some cases, Provincial or Territorial Governments.
Finding Common Ground
The nature and scope of the marine mammal research projects undertaken by co-management bodies and DFO in northern regions varies extensively with some partnerships and collaborations extending back to the mid 1980s such as the FJMC in the Western Arctic. Despite this diversity, it is evident that some common research themes have emerged across the various settlement areas in recent years including, but not limited to, the following: 1) the development of hunter/harvest-based biological monitoring programs for marine mammal subsistence species that are important from both a cultural and ecological perspective; 2) improving our mutual understanding of marine mammal seasonal movements and identification of critical habitats; 3) understanding and monitoring the effects of climate change on marine mammals and their habitats; 4) assessing potential impacts of industry activity on key species; and 5) building and developing research capacity, science-based education and decision-making expertise at the community level.
The FJMC in the Western Arctic was the first co-management body to adopt a hunter-based biological monitoring program to address both contemporary community issues and to fulfill the need for long-term marine mammal studies. The Committee and DFO researchers recognized that the subsistence harvest of marine mammals by Inuvialuit beneficiaries could provide long-term biological data that would otherwise be very expensive and logistically challenging to collect. Ongoing cooperative projects in the Inuvialuit Settlement Region include monitoring of both the ringed seal subsistence harvest (to examine body condition and reproduction; contaminant and disease loads), and the beluga whale harvest (to document size and sex of landed whales; timing of harvests; size of harvests). The Nunavut community-based monitoring network has developed into a consistent annual program within the greater Hudson Bay region since 2003. Nunavumiut hunters primarily collect seal (ringed, bearded, harp, harbour) and beluga and narwhal whale tissue samples as well as key marine mammal prey species. Since 2008, the TJFB has supported the Labrador biological collection program for ringed seals based out of the communities of Nain, Makkovik, Hopedale and Rigolet. Similar programs are likely to be undertaken in Nunavik once the newly created NMRWB is fully established. These monitoring programs engage and involve community members in all aspects of monitoring, and sample collection - from planning and coordination to communicating and interpreting data.

A hunter from Arviat, Hudson Bay is pictured giving a presentation to local elementary school children explaining how he coordinates the collection of ringed seal samples from local hunters in the area and then examines them (ringed seal foetus shown).
Photo: DFO
Understanding the seasonal movements of marine mammal species is necessary to properly assess population abundance, identify stocks or management units, identify critical habitats and ultimately develop long-term management and conservation strategies. Co-management bodies in all settlement areas have recognized this and partner with DFO on a variety of marine mammal satellite tagging programs (e.g. ringed seals, beluga whales, narwhal, bowhead whale). The contributions by hunters to these programs with regard to the selection of locations and times best suited for live captures, input on capture techniques, expertise in capture and handling animals and knowledge of animal behaviour have largely determined their success. Some of the results have surprised both hunters and DFO researchers and require new interpretations of migratory and diving behaviour as well as habitat use for some species. Presently there are experienced marine mammal tagging teams in Ulukhaktok, Tuktoyaktuk and Aklavik in the Western Arctic, Sanikiluaq, Igloolik and Kuujjuaraapik in the Hudson Bay region, and Nain and the Lake Melville area in Labrador.
The impacts of climate change on marine mammals, particularly those that are of cultural, nutritional and commercial importance is a major concern for co-management bodies, communities and the Department. Long-term data collected by the various hunter-based monitoring programs in all the settlement areas are presently being analyzed from the perspective of climate mediated changes in the marine environment (e.g. changes in sea ice coverage and snow accumulation) as are historical scientific data and local ecological knowledge regarding changes in animal distribution and abundance trends. These research approaches are providing species-specific 'snapshots' of change but don't adequately address how the dynamics of the ecosystem are being affected. In the Hudson Bay region of Nunavut, research to describe the complete food web in the Bay is being developed and will be used to build a model of trophic interactions from marine mammals down to nutrients and phytoplankton. Once this model is running it will be able predict how perturbations in the system, (e.g. effects of warmer water on key forage fish) may ultimately affect marine mammals. Predicting how to mitigate impacts of Arctic climate change on marine mammals, such as protection of seasonally critical habitats, or identification of the most vulnerable populations is relevant to the conservation of these species. Further, such information can be used by Northerners who will also need to adapt to preserve cultural and economic relevance of marine mammals in their communities.

Ringed seal satellite tagging team preparing to attach a tag on a juvenile ringed seal captured in the Lake Melville area, Labrador.
Photo: DFO

Members of the Paulatuk satellite tagging team in the Western Arctic release a ringed seal.
Photo: DFO

A Hunter from the Hudson Bay area touring the DFO Freshwater Institute's marine mammal laboratory.
Photo: DFO
The potential negative impacts of large scale industrial developments in northern regions are another major concern for co-management partners. The FJMC and DFO researchers in the western Arctic have conducted a number of collaborative studies examining the potential effects of oil and gas production in the Beaufort Sea, including the following more recent projects: effects of winter drilling activity on the distribution and movements of ringed seals (satellite tagging); the distribution, movements and behaviour of bowhead whales during summer feeding in the southeast Beaufort Sea (aerial surveys and satellite tagging); and distribution of beluga whales in the Mackenzie River estuary and adjacent offshore areas (aerial surveys, acoustic monitoring). With the prospect of oil and gas exploration in Baffin Bay and Lancaster Sound, new large scale mining proposals involving winter shipping and hydro electrical projects in the Hudson Bay region and both hydro electrical and oil and gas developments proposed along coastal Labrador, it is likely the impacts of these activities will be a concern to communities and the Department in the future.
The opportunities for hunters and other community members to learn new technical and leadership skills, to participate in all phases of a research program and to contribute their local ecological knowledge in an important and relevant manner is evident in the wide variety of programs briefly described here. Also, as previously mentioned, the success of many programs has been a direct result of hunter and/or community involvement. Throughout all the settlement areas there has be a concerted effort, some quite innovative, to improve communication and the flow of all types information including new opportunities for knowledge exchange among the co-management bodies, communities and the Department. For example, maps and animations of seal movements and locations (sometime in real time) from the satellite telemetry projects near Sanikiluaq, Hudson Bay, Amundsen Gulf, Beaufort/Chukchi Seas, and Lake Melville, Labrador can be viewed on internet. Posting these results on the web makes them available to the scientific community, media, northern communities, the public, students, and teachers in a timely manner. Looking to the future, both the Department and co-management bodies will continue to work closely to ensure the sustainable use and conservation of marine mammal resources in Canada's northern regions.
5.0 Species at Risk
5.1 British Columbia's killer whales and ocean disposal practices in Critical Habitat (Peter S. Ross)
The northern and southern populations of resident killer whales in British Columbia are listed as threatened and endangered, respectively, under the Species at Risk Act (SARA), which aims to protect species at risk from being killed or harmed (section 32) and protects any part of their Critical Habitat from destruction (section 58). These resident killer whales have been found to be contaminated with very high levels of polychlorinated biphenyls (PCBs). As hydrophobic chemicals, PCBs are either incorporated into the lipid fraction of the food web, where they biomagnify, or they attach to particles and sediments. High concentrations of PCBs encountered in harbours and industrial areas can provide a source to adjacent food webs.
Environment Canada regulates disposal at sea operations under the Canadian Environmental Protection Act 1999 (CEPA 1999; Disposal at Sea Regulations, Regulations Respecting Applications for Permits for Disposal at Sea). DFO is responsible for the issuance of SARA permits for activities that may harm a listed species or destroy their Critical Habitat. A PCB food web modeling tool was developed to explore disposal scenarios, and to support risk management practices related to killer whales and their Critical Habitat.
Disposal of materials with less than ambient sediment PCB concentrations at a receiving site is not expected to increase PCB delivery to killer whales. Disposal of materials containing greater than ambient PCB levels would benefit from an alternative strategy, including disposal at a site with high natural sedimentation rate, as long as this new site is deemed to be depositional and dredge materials are not further dispersed. This modeling effort suggests that a target sediment PCB range of 0.012 to 0.200 μg•kg-1 dry weight would optimally protect long-lived, high trophic level killer whales. However, these protective sediment PCB values are currently exceeded in many parts of coastal BC, underscoring the legacy left by the PCBs used decades ago.
An additional understanding of PCB pathways in coastal waters, including sources, sinks, sedimentation rates, and substrate types in both dredge and disposal sites will better inform risk-based decisions regarding the likely fate and consequences of disposal activities within killer whale Critical Habitat.
6.0 Publications 2009-2011
- Addison, R.F., D.C.G. Muir, M.G. Ikonomou, L. Harwood and T.G. Smith. 2009. Hexachlorocyclohexanes (HCH) in ringed seal (Phoca hispida) from Ulukhaktok (Holman), T: trends from 1978 to 2006. Science of the Total Environment 407 (2009), pp. 5139-5146. 10.1016/j.scitotenv
- Alava, J.J., Ikonomou, M.G., Ross, P.S., Costa, D., Salazar, S., Aurioles-Gamboa, D., and Gobas, F.A.P.C. 2009. Polychlorinated biphenyls and polybrominated diphenyl ethers in Galapagos sea lions (Zalophus wollebaeki). Environ.Toxicol.Chem. 28: 2271-2282.
- Alava, J.J., Ross, P.S., Ikonomou, M.G., Cruz, M., Jimenez-Uzcategui, G., Dubetz, C., Salazar, S., Costa, A.N., Villegas-Amtmann, S., Howorth, P., and Gobas, F.A.P.C. 2011a. DDT in endangered Galapagos sea lions (Zalophus wollebaeki). Mar.Pollut.Bull. 62: 660-671.
- Alava, J.J., Salazar, S., Cruz, M., Jimenez-Uzcategui, G., Villegas-Amtmann, S., Paez-Rosas, D., Costa, A.N., Ross, P.S., Ikonomou, M.G., and Gobas, F. 2011b. DDT strikes back: Galapagos sea lions face increasing health risks. Ambio 40: 425-430.
- Andersen, J. M., Y. F. Wiersma, G. Stenson, M. O. Hammill and A. Rosing-Asvid. 2009. Movement Patterns of Hooded Seals (Cystophora cristata) in the Northwest Atlantic Ocean during the Post-Moult and Pre-Breed Seasons. J. Northw. Atl. Fish. Sci., Vol. 42: 1–11.
- Anderwald, P., Daníelsdóttir, A.K., Haug, T., Larsen, F., Lesage, V., Reid, R., Víkingsson, G.A., Hoelzel, A.R. 2011. Possible cryptic stock structure for minke whales in the North Atlantic; implications for conservation and management. Biological Conservation 144: 2479-2489.
- Applebee, A., A.R. Thompson, L.N. Measures and M.E. Olson. 2010. Giardia and Cryptosporidium in harp and hooded seals from the Gulf of St. Lawrence, Canada. Veterinary Parasitology 173:19-23.
- Archambault, P., Snelgrove, P., Fisher, J.A.D., Gagnon, J.-M., Garbary, D. J., Harvey, M., Kenchington, E., Lesage, V., Levesque, M., Lovejoy, C., Mackas, D. McKindsey, C.W., Nelson, J., Pépin, P., Piché, L., Poulin, M. 2010. From Sea to Sea: Canada’s Three Oceans of Biodiversity. PLoS ONE 5(8):e12182. doi:10.1371/journal.pone.0012182.
- Asselin, N.C. and Richard, P.R. 2011. Results of narwhal (Monodon monoceros) aerial surveys in Admiralty Inlet, August 2010. CSAS Research Document 2011/056: 30 p.
- Asselin, N.C., Barber, D.G. Stirling, I., Ferguson, F.H. and Richard, P.R. 2011. Beluga (Delphinapterus leucas) habitat selection in the eastern Beaufort Sea in spring, 1975-1979. Polar Biology DOI 10.1007/s00300-011-0990-5: 16 p.
- Bahoura, M, and Simard, Y. 2009. Blue whale calls classification using short-time Fourier and wavelet packet transforms and artificial neural network. Digital Signal Processing 20: 1256-1263. doi:10.1016/j.dsp.2009.10.024.
- Bailleul, F., Lesage, V. and M. O. Hammill. 2010. Spherical First-Passage Time: a tool to investigate area-restricted search in three-dimensional movements. Ecological Modelling 221: 1665-1673.
- Bajzak, C., M.O.Hammill, G.B.Stenson and S.Prinsenberg. 2011. Drifting away: implications of changes in ice conditions for a pack ice-breeding phocid, the harp seal (Pagophilus groenlandicus).Can. J.Zool.89(11):1050-1062.
- Bajzak, C.E., S.D. Côté, M.O. Hammill, G. Stenson. 2009 . Intersexual differences in the postbreeding foraging behaviour of the Northwest Atlantic hooded seal. Marine Ecology Progres Series 385:285-294.
- Barlow, J., Calambokidis, J., Falcone, E.A., Baker, C.S., Burdin, A.M., Clapham, P.J., Ford, J.K.B., Gabriele, C.M., LeDuc, R., Matilla, D.K., Quinn, T.J. II, Rojas-Bracho, L., Straley, J.M., Taylor, B.L., Urbán R., J., Wade, P., Weller, D., Witteveen, B.H., Yamaguchi, M. 2011. Humpback whale abundance in the North Pacific estimated by photographic capture-recapture with bias correction from simulation studies.Marine Mammal Science 27(4):793-818.
- Bell, R.K., and Harwood, L.A. In press. Harvest-based Monitoring in the Inuvialuit Settlement Region: Steps for Success. Arctic.
- Benjamins, S., Kulka, D.W., and Lawson, J.W. 2010. Recent incidental catch of sharks in gillnet fisheries of Newfoundland and Labrador, Canada. Endangered Species Research 11: 133-146.
- Bennett, E.R., Ross, P.S., Huff, D., Alaee, M., and Letcher, R.J. 2009. Chlorinated and brominated organic contaminants and metabolites in the plasma and diet of a captive killer whale (Orcinus orca). Mar.Pollut.Bull. 58: 1078-1095.
- Benoit, D., Simard, Y., Gagné, J.A., Geoffroy, M. and Fortier, L. 2010. From polar night to midnight sun: photoperiod, seal predation, and the diel vertical migrations of polar cod (Boreogadus saida) under landfast ice in the Arctic Ocean. Polar Biology 33(11): 1505-1520. DOI 10.1007/s00300-010-0840-x
- Benoît, H.P., Swain, D.P., Bowen, W.D., Breed, G.A., Hammill, M.O., Harvey, V. 2011. Evaluating the potential for grey seal predation to explain elevated natural mortality in three fish species in the southern Gulf of St. Lawrence. Marine Ecology Progress Series 442:149-167.
- Benoît, H. P., Swain, D. P., and Hammill, M.O. 2011. Seasonal patterns in the spatial overlap of southern Gulf of St. Lawrence cod and grey seals, with a discussion of sources of error and possible bias. DFO Can. Sci. Adv. Sec. Res. Doc. 2011/018.
- Benoit, H.P., M.O. Hammill and D.P. Swain. 2011. Estimated consumption of southern gulf of St. Lawrence cod by grey seals:bias, uncertainty and two proposed approaches. CSAS Res. Doc. 2011/041
- Bowen, W. D. 2011. Historical Grey Seal Abundance and Changes in the Abundance of Grey Seal Predators in the Northwest Atlantic. DFO Can. Sci. Advis. Sec. Res. Doc. 2011/026.
- Bowen, W. D. and Iverson, S. J. 2011. Sources of Bias and Uncertainty in Seal Diet Composition: Hard Part and Fatty Acid Analysis. DFO Can. Sci. Advis. Sec. Res. Doc. 2011/025.
- Bowen, W. D. and Lidgard, D. 2011. Vertebrate Predator Control: Effects on Prey Populations in Terrestrial and Aquatic Ecosystems. DFO Can. Sci. Advis. Sec. Res. Doc. 2011/028.
- Bowen, W. D. and Northridge, S. 2010. Morphometrics, age estimation, and growth, p. 98-118. In Boyd, I. L., Bowen, W. D., and Iverson, S. J. (eds.) Marine Mammal Ecology and Conservation: A handbook of techniques, Oxford University Press.
- Bowen, W. D., Baker, J. D., Boyd, I. L., Estes, J. A., Ford, J. K. B., Kraus, S. D., Siniff, D., Stirling, I., and Wells, R. S. 2010. Long-term studies, p. 283-305. In Boyd, I. L., Bowen, W. D., and Iverson, S. J. (eds.) Marine Mammal Ecology and Conservation: A handbook of techniques, Oxford University Press, Oxford, U. K.
- Bowen, W. D., Beck, C. A., and Austin, D. 2009. Pinniped ecology, p. 852-860. In Encyclopedia of Marine Mammals. Second Edition. W. F. Perrin, B. Wursig, and H. G. M. Thewissen (eds.) Academic Press
- Bowen, W. D., Hammill, M. O., Koen-Alonso, M., Stenson, G., Swain, D.P., and Trzcinski K. 2009. Proceedings of the National Workshop on the Impacts of Seals on Fish Populations in Eastern Canada (Part 2) CSAS, Proceedings 2009/020
- Bowen, W. D., P. Carter, and M. Hammill. 2011. Estimated Grey Seal Diets near Sable Island Derived from Fecal Samples: 1991 to 2010. DFO Can. Sci. Advis. Sec. Res. Doc. 2011/024: iv+7p.
- Bowen, W.D., C. E. den Heyer, J. I. McMillan and M. Hammill. 2011. Pup production at Scotian Shelf grey seal (Halichoerus grypus) colonies in 2010. DFO Can. Sci. Advis. Sec. Res. Doc. 2011/066
- Boyd, I. L., Bowen, W. D., and Iverson, S. J. 2010. Marine Mammal Ecology and Conservation: A handbook of techniques, Oxford University Press. 450p.
- Breed, G. A., I. D. Jonsen, R. A. Myers, W. D. Bowen, and M. L. Leonard. 2009. Sex-specific, seasonal foraging tactics in adult grey seals (Halichoerus grypus) are revealed by behaviour discriminating state-space models. Ecology 90:3209-3221.
- Breed, G. D., Bowen, W. D. and Leonard, M. L. 2011. Development of foraging strategies with age in a long-lived marine predator. Mar. Ecol. Prog. Ser. 431:267-279.
- Brillant, S., and E.A. Trippel. 2010. Elevations of lobster fishery groundlines in relation to their potential to entangle endangered North Atlantic right whales in the Bay of Fundy, Canada. ICES J. Mar. Sci. 67: 355-364.
- Buckman, A.H., Veldhoen, N., Ellis, G., Ford, J.K.B., Helbing, C.C. and Ross, P.S. 2011. PCB-associated changes in mRNA expression in killer whales (Orcinus orca) from the NE Pacific Ocean. Environmental Science &Technology 45(23):10194-202.
- Burns, J.M., N. Skomp, N. Bishop, K. Lestyk, and M. Hammill. 2010. Development of aerobic and anaerobic metabolism in cardiac and skeletal muscles from harp and hooded seals. The Journal of Experimental Biology 213:740-748
- Calambokidis, J., Barlow, J., Ford, J.K.B., Chandler, T.E., and Douglas, A.B. 2009. Insights into the population structure of blue whales in the eastern North Pacific from recent sightings and photographic identification. Marine Mammal Science 25:816-832
- Chambellant, M. and S.H. Ferguson. 2009. Ageing live ringed seals (Phoca hispida): which 1 tooth to pull? Marine Mammal Science 25: 478-486. DOI: 10.1111/j.1748-7692.2008.00269.x
- Chassot, E., D. Duplisea, M.O. Hammill, A. Caskenette, N. Bousquet, Y. Lambert, and G. Stenson. 2009. The role of predation by harp seals (Pagophilus groenlandicus) in the collapse and non-recovery of northern Gulf of St. Lawrence cod (Gadus morhua). Marine Ecology Progress Series 379: 279-297.
- Christensen, J.R., Letcher, J.R., and Ross, P.S. 2009. Persistent or not persistent? Polychlorinated biphenyls are readily depurated by grizzly bears (Ursus arctos horribilis). Environ.Toxicol.Chem. 28: 2206-2215.
- Clark, D.S., Coopper, T., Doherty, A., Ford, J.K.B., Koops, M.A., Mandrak, N.E., Morris, T.J., and Smedbol, R.K. 2009. Terms and Concepts Used in the SARA Program. Canadian Science Advisory Secretariat, Res. Doc 2009/082. vi + 33 p.
- Crawford, W.R. and J.R. Irvine. 2011. State of physical, biological, and selected fishery resources of Pacific Canadian marine ecosystems in 2010. DFO Can. Sci. Advis. Sec.Res. Doc. 2011/054. x + 163 p.
- Cullon, D.L., Yunker, M.B., Alleyne, C., Dangerfield, N.J., O’Neill, S., Whiticar, M.J., and Ross, P.S. 2009. Persistent organic pollutants in chinook salmon (Oncorhynchus tshawytscha): implications for resident killer whales of British Columbia and adjacent waters. Environ.Toxicol.Chem. 28: 148-161.
- Deecke, V.B., Barrett-Lennard, L.G., Spong, P., and Ford, J.K.B. 2010. The structure of stereotyped calls reflects kinship and social affiliation in resident killer whales (Orcinus orca). Naturwissenschaften 97:513-518.
- Desforges, J.P., Ross, P.S., Loseto, L.L. 2012.Transplacental transfer of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in arctic beluga whales (Delphinapterus leucas). Environmental Toxicology and Chemistry 31(2):296-300.
- DFO. 2011. Recovery Potential Assessment for Northern Bottlenose Whales (Hyperoodon ampullatus) in Canada. DFO Can. Sci. Advis. Sec. Sci. Advis. Rep. 2011/031
- DFO. 2011. Impacts of Grey Seals on Fish Populations in Eastern Canada. DFO Can. Sci. Advis. Sec. Sci. Advis. Rep. 2010/071.Diemer, KM, MJ Conroy, SH Ferguson, DDW Hauser, A Grgicak-Mannion, AT Fisk. 2011. Marine mammal and seabird summer distributions and abundances in the fjords of northeast Cumberland Sound, Baffin Island. Polar Biology 34: 41-48. DOI 10.1007/s00300-010-085
- Doniol-Valcroze, T, Hammill, M. O. and Lesage, V. 2010. Harvest advice for Eastern Hudson Bay beluga (Delphinapterus leucas). DFO Can. Sci. Advis. Sec. Res. Doc. 2010/121. iv + 13 p.
- Doniol-Valcroze, T., Hammill, M.O., Lesage, V. 2011. Information on abundance and harvest of eastern Hudson Bay beluga (Delphinapterus leucas). DFO Can.Sci.Advis.Sec., Res.Doc.2010/121. iv + 13 p.
- Doniol-Valcroze, T., Lesage, V., Giard, J., Michaud, R. 2011. Optimal foraging theory predicts diving and feeding strategies of the largest marine predator. Behavioural Ecology 22(4): 880-888.
- Dufresne, M.M., Frouin, H., Pillet, S., Hammill, M.O., Lesage, V., De Guise, S., and Fournier, M. 2010. Comparative sensitivity of two species of seals to several environmental contaminants using in vitro exposure. Marine Pollution Bulletin 60: 344-349.
- Earle, J. A., M. M. Melia, N. V. Doherty, O. Nielsen, and S. L. Cosby. 2010. Identification of Phocine Distemper Virus in Seals off the East Coast of USA in 2006: Genetic Comparison with Viruses from the 1988 and 2002 Epizootics. Emerging Infectious Diseases Vol. 17 215-220.
- Ferguson, S.H. 2010. MMR Freeman and L Foote (eds): Inuit, polar bears, and sustainable use: local national and international perspectives. Human Ecology 38: 463-465.
- Ferguson, S.H. 2010. Review of aerial survey estimates for ringed seals (Pusa hispida) in western Hudson Bay, 2009 and 2010. DFO Can. Sci. Advis. Sec. Sci. Res. Doc. 2010/xxx.
- Ferguson, S.H., B.G.Young. 2011. Aerial survey estimates of hauled-out ringed seal (Pusa hispida) density in western Hudson Bay, June 2009 and 2010. DFO Can. Sci. Advis. Sec. Res. Doc. 2011/029. iv + 12 p.
- Ferguson, S.H., M.C.S.Kingsley and J.W. Higdon. 2012. Killer whale (Orcinus orca) predation in a multi-prey system. Population Ecology 54(1):31-41.
- Ferguson, SH, L Dueck, LL Loseto, SP Luque. 2010. Bowhead whale (Balaena mysticetus) seasonal selection of sea ice. Marine Ecology Progress Series 411: 285-297.
- Ferreira, E.O., L.L. Loseto, S.H. Ferguson.2011. Assessment of growth-layer groups from ringed seal (Pusa hispida) as biomonitors of inter- and intra-annual Hg, d15N and d13C variation. Can. J. Zool. 89: 774-784.
- Forbes, L.B., L. Measures and A. Gajadhar. 2009. Infectivity of Toxoplasma gondii in northern traditional (country) foods prepared with meat from experimentally infected seals. Journal of Food Protection 72: 1756-1760.
- Ford J.K.B., Rambeau A.L., Abernethy R.M., Boogaards M.D., Nichol L.M., and Spaven L.D. 2009. An Assessment of the Potential for Recovery of Humpback Whales off the Pacific Coast of Canada. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/015. iv + 33 p.
- Ford, J.K.B, G.M. Ellis, P.F. Olesiuk, and K.C. Balcomb. 2010. Linking killer whale survival and prey abundance: food limitation in the oceans’ apex predator? Biology Letters, 6:139-142. Published on-line before print September 15, 2009.
- Ford, J.K.B, Wright, B.M., Ellis, G.M., and Candy, J.R. 2010. Chinook salmon predation by resident killer whales: seasonal and regional selectivity, stock identity of prey, and consumption rates. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/101. iv + 43 p.
- Ford, J.K.B. 2009. Killer whale Orcinus orca. In: Perrin, W.F., Wursig, B., and J.G.M. Thewissen (eds.), The Encyclopedia of Marine Mammals Second Edition. Elsevier, New York, NY. P. 650-657.
- Ford, J.K.B. 2009. Dialects. In: Perrin, W.F., Wursig, B., and J.G.M. Thewissen (eds.), The Encyclopedia of Marine Mammals Second Edition. Elsevier, New York, NY.
- Ford, J.K.B., B. Koot, S. Vagle, N. Hall-Patch, and G. Kamitakahara. 2010. Passive acoustic monitoring of large whales in offshore waters of British Columbia. Can. Tech. Rep. Fish. Aquat. Sci. 2898: v + 30 p.
- Ford, J.K.B., G.M. Ellis, C.O. Matkin, M.H. Wetklo, L.G. Barrett-Lennard, and R.E. Withler. 2011. Shark predation and tooth wear in a population of northeastern Pacific killer whales. Aquatic Biology 11:213-224.
- Ford, J.K.B., R.M. Abernethy, A.V. Phillips, J. Calambokidis, G.M. Ellis and L.M. Nichol. 2010. Distribution and relative abundance of cetaceans in western Canadian waters from ship surveys, 2002-2008. Can. Tech. Rep. Fish. Aquat. Sci. 2913: v + 51 p.
- Frie, A.K., K.-A. Fagerheim, M.O. Hammill, F.O. Kapel, C. Lockyer, G.B. Stenson, A. Rosing-Asvid and V. Svetochev. 2011. Error patterns in age estimation of harp seals (Pagophilus groenlandicus): results from a transatlantic, image-based blind-reading experiment using known-age teeth. ICES J. Mar. Sci. 68:1942-1953.
- Frouin H., Lebeuf M., Hammill M.O., Sjare B., and M.Fournier. 2011. PBDEs in serum and blubber of harbour, grey and harp seals pups from Eastern Canada. Chemosphere 82:663-669.
- Frouin, H, Lebeuf M, Hammill M, Masson S, Fournier M . 2010 Effects of individual polybrominated diphenyl ether (PBDE) congeners on harbour seal immune cells in vitro . Marine Pollution Bulletin 60: 291-298
- Frouin, H., L. Menard, L. Measures, P. Brousseau and M. Fournier. 2010. T lymphocyte-proliferative responses of a grey seal (Halichoerus grypus) exposed to heavy metals and PCBs in vitro. Aquatic Mammals 36: 365-371.
- Frouin, H., Lebeuf, M., Hammill, M., Fournier, M. 2010.Toxic effects of polybrominated diphenyl ethers (PBDEs) on harbour seal (Phoca vitulina) immune cells in vitro. Marine Pollution Bulletin 60 :291–298.
- Frouin, H., Lebeuf, M., Hammill, M., Fournier, M. 2010. Phagocytosis in pup and adult harbour, grey and harp seals. Veterinary Immunology and Immunopathology . 134:160-168.
- Frouin, H., Loseto, L., Stern, G., Haulena, M., and Ross, P.S. 2012. Mercury toxicity in beluga whale lymphocytes: Limited effects of selenium protection. Aquatic Toxicology 109: 185-193.
- Gaden A., S.H. Ferguson, L. Harwood, H. Melling, G.A. Stern. 2009. Mercury trends in ringed seals (Phoca hispida) from the western Canadian Arctic since 1973: associations with sea ice duration. Environmental Science and Technology 43: 3646-3651. DOI: 10.
- Garrett, C. and Ross, P. S. 2010. Recovering resident killer whales: A guide to contaminant sources, mitigation, and regulations in British Columbia. Fisheries and Oceans Canada. Canadian Technical Report of Fisheries and Aquatic Sciences No. 2894. 1-224.
- Gervaise, C., Barazzuti, A., Busson, S., Simard, Y., and Roy, N. 2010. Automatic detection of bioacoustics impulses based on kurtosis under weak signal to noise ratio. Applied Acoustics 71: 1020-1026. DOI: 10.1016/j.apacoust.2010.05.009
- Gervaise, C., Stephan, Y., and Simard, Y. 2010. On the use of time-frequency methods in a passive acoustic monitoring system, in Proceedings of ’2010 IEEE International conference on Acoustics, Speech and Signal Processing’. Dallas, Tx, USA. 14-19 March.
- Givens, G. H., Ryan M. Huebinger, John C. Patton, Lianne D. Postma, Melissa Lindsay, Robert S. Suydam, John C. George, Cole W. Matson, J. W. Bickham. 2010. Population genetics of bowhead whales (Balaena mysticetus) in the western Arctic. Arctic 63: 1-12.
- Gosselin, J.-F., Lesage, V., Hammill, M.O. 2009. Index estimates of abundance for beluga in eastern Hudson Bay, James Bay, and Ungava Bay in summer 2008. CSAS Doc. Res. 2009/006: 25 p. Available at http://www.dfo-mpo.gc.ca/csas
- Gosselin, J-F. Lesage, V. et Hammill, MO. 2009. Abundance indices of beluga in James Bay, eastern Hudson Bay and Ungava Bay in 2008. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/006. iv + 25 p.
- Grant, P.B.C., Johannessen, S.C., Macdonald, R.W., Yunker, M., Sanborn, M., Dangerfield, N., Wright, C., and Ross, P.S. 2011. Environmental fractionation of PCBs and PBDEs during particle transport as recorded by sediments in coastal waters. Environ.Toxicol.Chem. 30: 1522-1532.
- Grebner, D.M., Parks, S.E., Bradley, D.L., Miksis-Olds, J.L., Capone, D.E., and Ford, J.K.B. 2011. Divergence of a stereotyped call in northern resident killer whales. J. Acoustical Society America 129:1067-1072.
- Grist, J., S. Josey, L. Boehme, M. Meredith, F.J.M. Davidson, G. Stenson and M.O.Hammill. 2011. Temperature signature of high latitude Atlantic boundary currents revealed by marine mammal-borne sensor and Argo data. Geophysical Research Letters 38:L15601. 6pp.
- Haarr, M.L., L.D. Charlton, J.M. Terhune and E.A. Trippel. 2009. Harbour porpoise (Phocoena phocoena) presence patterns at an aquaculture cage site in the Bay of Fundy, Canada. Aquat. Mamm. 35: 203-211.
- Hammill, M.O., Bowen, W.D.and B. Sjare. 2010. Status of harbour seals (Phoca vitulina) in Atlantic Canada. In: G.Desportes, A. Bjorge, A. Rosing-Asvid, G. Waring and M. Acquarone (eds.) NAMMCO Scientific Publications, Volume 8:175-190.
- Hammill, M. O. Kingsley, M. C. S. Lesage and Gosselin, J.-F. 2009. Abundance of Eastern Hudson Bay belugas. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/009. iv + 22 p.
- Hammill, M. O., Bowen, W. D., and Blanchard, W. 2009. Timing of grey seal pupping on Hay Island. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/091.Hammill, M.O. 2009. Earless seals (Phocidae). W.F. Perrin, B. Wursig and J.G.M. Thewissen (eds). Pages 342-348, In Encyclopedia of Marine Mammals (2nd ed). Elsevier New York. 1316 pp.
- Hammill, M.O. 2009. Ringed seal (Pusa hispida). W.F. Perrin, B. Wursig and J.G.M. Thewissen (eds). Pages 342-348, In Encyclopedia of Marine Mammals (2nd ed). Elsevier Boston. 1316 pp.
- Hammill, M.O. 2010. Feeding by grey seals in the southern Gulf of St Lawrence. CSAS Res Doc. 2010/130.
- Hammill, M.O. and D.P. Swain 2011. A controlled experiment to test the impact of removals of grey seals on the mortality of southern gulf cod. CSAS Res Doc. 2011/013.
- Hammill, M.O. and G.B. Stenson 2010. Comment on “Towards a precautionary approach to managing Canada’s commercial harp seal hunt” by Leaper et al. ICES J. Mar. Sci. 67:321-322.
- Hammill, M.O. and G.B. Stenson. 2009. A preliminary evaluation of the performance of the Canadian management approach for harp seals using simulation studies. CSAS Res Doc. 2009/093.
- Hammill, M.O. and G.B. Stenson. 2009. Abundance of northwest Atlantic harp seals (1952-2010). CSAS Res. Doc. 2009/114.
- Hammill, M.O. and G.B. Stenson. 2010. Pup production of Northwest Atlantic grey seals in the Gulf of St. Lawrence. Canadian Science Advisory Secretariat Res. Doc. 2010/122.
- Hammill, M.O. and G.B. Stenson. 2011. Estimating abundance of Northwest Atlantic harp seals, examining the impact of density dependence. Canadian Science Advisory Secretariat Res. Doc. 2011/011.
- Hammill, M.O. and M.C.S. Kingsley. 2009. Harvest advice for Eastern Hudson Bay belugas. CSAS Res Doc. 2009/89.
- Hammill, M.O., and Lesage, V. 2009. Seasonal movements and abundance of beluga in northern Quebec (Nunavik) based on weekly sightings information. CSAS Doc. Res. 2009/010: 14 p.
- Hammill, M.O., B. Ferland-Raymond, L.-P. Rivest and G. B. Stenson. 2009. Modelling Northwest harp seal populations: modifying an Excel model to R. CSAS Res. Doc. 2009/108.
- Hammill, M.O., W.D. Bowen and S. Sjare. 2011. Status of harbour seals in Atlantic Canada. NAMMCO Scientific publication . pages 175-189, In G. Desportes, a. Bjorne, A. Rosing-Asvid and G.T. Waring. Harbour seals in the North Atlantic and the Baltic. NAMMCO Scientific publications vol. 8.
- Hammill, M.O., W.D. Bowen and W. Blanchard. 2009. Timing of grey seal pupping on Hay Island. CSAS Res Doc 2009/91.
- Hammill, M.O. and G.B. Stenson. 2011. Modelling grey seal abundance in Canadian waters. 2011/014.
- Handegard, N.O., Demer, D.A., Kloser, R., Lehodey, P., Maury, O., and Simard, Y. 2009. Toward a global ocean ecosystem Mid-trophic Automatic Acoustic Sampler (MAAS). Community white paper. OceanObs’09 Venice, Italy. 8 p.
- Hanna, B., Cott, P.A., Joynt, A.A. and Harwood, L.A. 2012. Managing anthropogenic underwater noise in the Northwest Territories, Canada. In: Popper, A.N.and Hawkins, A. (eds.) The Effects of Noise on Aquatic Life. Advances in Experimental Medicine and Biology, Volume 730. Springer, NewYork. pp 625-627.
- Hanson, M.B., Baird, R.W., Ford J.K.B., Hempelmann-Halos, J., Van Doornik, D.M., Candy, J.R., Emmons, C.K., Schorr, G.S., Gisborne, B., Ayres, K.L., Wasser, S.K., Balcomb, K.C., Balcomb-Bartok, K., Sneva, J.G., and Ford, M.J. 2010. Species and stock identification of prey consumed by endangered southern resident killer whales in their summer range. Endangered Species Research 11:69-82.
- Harris, K.A., Nichol, L.M., and Ross, P.S. 2011. Hydrocarbon concentrations and patterns in free-ranging sea otters (Enhydra lutris) from British Columbia, Canada. Environ. Toxicol. Chem. 30(10):2184-93.
- Harris, K.A., Yunker, M.B., Dangerfield, N.J., and Ross, P.S. 2011. Sediment-associated aliphatic and aromatic hydrocarbons in coastal British Columbia, Canada: concentrations, composition, and associated risks to protected sea otters. Environ.Pollut. 159: 2665-2674.
- Harvey, V. and M.O. Hammill. 2010. Variations on spatial distribution on fish abundance in eastern Scotian shelf over the past four decades. CSAS Res Doc. 2010/132.
- Harvey, V. M.O. Hammill, W.D. Bowen. 2010. Spatial overlap between grey seals and their prey in 4VsW. CSAS Res. Doc. 2010/133
- Harvey, V., M.O. Hammill, D. Swain. 2010. Summer Overlap Between a Central-place Forager And Its Prey in the southern gulf of St. Lawrence CSAS Res Doc 2010/131.
- Harvey, V., M.O. Hammill, D.P. Swain, G.A. Breed, C. Lydersen, and K.M. Kovacs. 2010. Winter foraging by a top predator, the grey seal, in relation to the distribution of prey. CSAS Res. Doc. 2010/124
- Harwood, L. A. 2009. Status of anadromous Arctic charr (Salvelinus alpinus) of the Hornaday River, Northwest Territories, as assessed through community-based sampling of the subsistence fishery, August-September 1990-2007. Can. Ms. Rep. Fish. Aquat. Sci. 2890. vii + 34 p.
- Harwood, L.A. 2012. Marine Mammals. In: C. Burn (ed.) Herschel Island - Qikiqtaryuk: A Natural and Cultural History of Yukon's Arctic Island. Wildlife Management Advisory Council (North Slope), Whitehorse, Yukon.
- Harwood, L. A., Sandstrom, S., and Linn, E. 2009. Status of anadromous Dolly Varden (Salvelinus malma) of the Rat River, Northwest Territories, as assessed through sampling of the subsistence fishery (1995-2007). Can. Ms. Rpt. Fish. Aquatic Sci. 2891.
- Harwood, L.A., Smith, T.G., Auld, J.C. 2012. Fall migration of ringed seals (Phoca hispida) through the Beaufort and Chukchi seas, 2001-02. Arctic 65(1):35-44
- Harwood, L.A., Smith, T.G., Melling, H., Alikamik, J., and Kingsley, M.C.S. In press. Ringed Seals and Sea Ice in Canada’s Western Arctic: Harvest-Based Monitoring 1992–2011. Arctic.
- Harwood, L., Joynt, A.A., Kennedy, D., Pitt, R., and Moore, S. E. 2009. Spatial restrictions and temporal planning as measures to mitigate potential effects of seismic noise on cetaceans: a working example from the Canadian Beaufort Sea, 2007-2008. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/040. iv + 14 p.
- Harwood, L.A., and Joynt, A, A. 2009. Factors influencing the effectiveness of Marine Mammal Observers on seismic vessels, with examples from the Canadian Beaufort Sea. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/048. iv + 9 p.
- Harwood, L.A., Auld, J. C., Joynt, A. A., and Moore, S.E. 2010. Distribution of bowhead whales in the SE Beaufort Sea during late summer, 2007-2009. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/111. v+22 p.
- Harwood, L.A., Gordon, D.C. and Johnson, J. 2012. Fishes. In: C. Burn (ed.) Herschel Island - Qikiqtaryuk: A Natural and Cultural History of Yukon's Arctic Island. Wildlife Management Advisory Council (North Slope), Whitehorse, Yukon.
- Heide-Jørgensen, M.P., K.L. Laidre, Ø. Wiig, L. Postma, L.P. Dueck, and L. Bachmann. 2010. Large scale sexual aggregation of bowhead whales. Endangered Species Research 13: 73-78.
- Higdon J.W., D.D.W. Hauser and S.H. Ferguson. 2012. Killer whales (Orcinus orca) in the Canadian Arctic: Distribution, prey items, group sizes and seasonality. Marine Mammal Science 28(2):E93-E109.
- Higdon, J.W. and S.H. Ferguson. 2009. Loss of Arctic sea ice causing punctuated change in sightings of killer whales (Orcinus orca) over the past century. Ecological Applications 19: 1365-1375.
- Higdon, J.W., and S.H. Ferguson. 2011. Reports of humpback and minke whales in the Hudson Bay region, eastern Canadian Arctic. Northeastern Naturalist 18/3: 370-377.
- Himworth, C.G., M. Haulena, D. M. Lambourn, J.K. Gaydos, J. Huggins, J. Calambokidis, J.K.B. Ford, K. Zaremba, and S. Raverty. 2010. Pathology and epidemiology of phocid herpesvirus-1 infections in wild and rehabilitating harbor seals (Phoca vitulina) in the Northeastern Pacific. J. Wildl. Dis. 46:1046-1051.
- Huggins, F. E., S. A. Raverty, O. Nielsen, N. E. Sharp, J. D. Robertson, N. V. C. Ralston. An XAFS Investigation of Mercury and Selenium in Beluga Whale Tissues. Environmental Bioindicators 4:291-302.
- Hugues P. Benoît HP, Swain DP, Bowen WD, Breed GA, Hammill MO, Harvey V. In press. Can predation by grey seals explain elevated natural mortality in three fish species in the southern Gulf of St. Lawrence? Marine Ecology Progress Series 000:000-000.
- Iverson, S. J., Sparling, C. E., Williams, T., Lang, S.L.C., and Bowen, W. D. 2010. Measurement of individual and population energetics of marine mammals, p. 191-221. In Boyd, I. L., Bowen, W. D., and Iverson, S. J. (eds.) Marine Mammal Ecology and Conservation: A handbook of techniques, Oxford University Press.
- Kelly, T.C., Loseto, L.L., Stewart, R.E.A., Yurkowski, M., and Ferguson, S.H. 2010. Importance of Eating Capelin: Unique Dietary Habits of Hudson Bay Beluga. Pages 53-69 in Ferguson, SH, Loseto LL, Malory, ML (editors). 2010. A little less Arctic: changes to top predators in the world’s largest northern inland sea, Hudson Bay. Springer Publishing Company.
- Kingsley, M.C.S., Richard, P. and Ferguson, S.H. 2012. Stock-dynamic model for the northern Hudson Bay narwhal population based on 1982-2008 aerial surveys. DFO Can. Sci. Advis. Sec. Res. Doc. 2012/020. iv + 20 p.
- Klauda, R. J., M. J. Dadswell, R. A. Cunjak, J. E. Cooper, K. L. Beal, and T. S. Avery, editors. 2009. Challenges for Diadromous Fishes in a Dynamic Global Environment. American Fisheries Society, Symposium 69, Bethesda, Maryland.
- Lachmuth, C.L., Alava, J.J. , Hickie, B.E., Johannessen, S.C., Macdonald, R.W., Ford, J.K.B., Ellis, G.M. Gobas, F.A.P.C, and Ross, P.S. 2010. Ocean disposal in resident killer whale (Orcinus orca) Critical Habitat: science in support of risk management. Fisheries and Oceans Canada. Canadian Science Advisory Secretariat: Research Document No. 2010/116.
- Laidre, K., Heide-Jørgensen, M.P., Stern, H, Richard, P. 2012. Unusual narwhal sea ice entrapments and delayed autumn freezeup trends Polar Biology 35:149-154.
- Lang, S. L. C., D. J. Boness, W. D. Bowen and S. J. Iverson. 2010. Primiparous females do not exhibit reduced maternal care in gray seals (Halichoerus grypus). Mar. Mamm. Sci. DOI: 10.1111/j.1748-7692.2010.00443.x
- Lang, S. L. C., Iverson, S. J. and Bowen, W. D. 2009. Repeatability in lactation performance and the consequences for maternal reproductive success in grey seals. Ecology 90:2513-2523.
- Lang, S. L. C., Iverson, S. J. and Bowen, W. D. 2011. The influence of reproductive experience on milk energy output and lactation performance in the grey seal (Halichoerus grypus). PlosOne 6: 1-11, e19487.
- Larrat, S., S. Lair and L. Measures. 2012. Rakemarks on a harbour porpoise (Phocoena phocoena) calf suggestive of a fatal interaction with an Atlantic white-sided dolphin (Lagenorhynchus acutus). AquaticMammals 38(1):86-91.
- Lawson, J.W. 2009. Perception bias corrections for abundance estimates of cetaceans in Newfoundland waters in during the 2007 T-NASS survey SC/17/AE /14, NAMMCO, Quebec City, 7-9 October 2009.
- Lawson, J.W. 2009. The use of sound propagation models to determine safe distances from a seismic sound energy source, DFO Can. Sci. Advis. Sec. Res. Doc., Ottawa, ON. 2009/060. iv + 15p.
- Lawson, J.W., Gosselin, J.-F., Desportes, G., Acquarone, M., Heide-Jørgensen, M.P., Mikkelsen, B., Pike, D., Víkingsson, G., Zabavnikov, V., and Øien, N. 2009. A note on the distribution of short-beaked common dolphins, Delphinus delphis, observed during the 2007 T-NASS (Trans North Atlantic Sightings Survey), International Whaling Commission, Copenhagen, DK. SC/61/SM35. 4p.
- Lawson. J.W., and Gosselin, J.-F. 2009. Distribution and preliminary abundance estimates for cetaceans seen during Canada’s marine megafauna survey - A component of the 2007 TNASS. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/031. vi + 28 p.
- Lebeuf, M., Trottier, S., Noël, M., Raâch, M. and Measures, L. 2009. A twenty year (1987-2007) trend of PBDEs in beluga from the St. Lawrence Estuary, Canada. Dioxin 2009, Organohalogen Compounds 71: 372-376.
- Lenky C, and B. Sjare. (2011). Changes in seal habitat use of nearshore waters around Newfoundland and Sourthern Labrador: implications for potential predation on salmon. The Open Conservation Biology Journal (5)13-24. DOI 10.2174/1874839201105010013
- Lesage, V, Y Morin, È Rioux, C Pomerleau, SH Ferguson, and É Pelletier. 2010. Stable isotopes and trace metals as indicators of diet and habitat use in cetaceans: predicting errors related to preservation, lipid extraction and normalization. Marine Ecology Progress Series 419:249-265.
- Lesage, V., Baillargeon, D., Turgeon, S., and Doidge, D.W. 2009. Harvest statistics for beluga in Nunavik, 2005-2008. CSAS Doc. Res. 2009/007: 21 p.
- Lestyk, K., L. Folkow, A. Blix , M.O. Hammill, J. Burns. 2009. Development of myoglobin concentration and acid buffering capacity in harp (Pagophilus groenlandicus) and hooded (Cystophora cristata) seals from birth to maturity. J. Comp. Physiol. B. 179:985-996
- Lewis, A., M.O. Hammill, Power, D.W. Doidge and V. Lesage. 2009. Movement and aggregation of Eastern Hudson Bay beluga whales (Delphinapterus leucas): a comparison of patterns found through satellite telemetry and Nunavik traditional ecological knowledge. Arctic 62:13-24.
- Loseto, L., T. Wazny, H. Cleator, B. Ayles, D. Cobb, L. Harwood, C. Michel, O. Nielsen, J. Paulic, L. Postma, P. Ramlal, J. Reist, P. Richard, P.S. Ross, S. Solomon, W. Walkusz, L. Weilgart and B. Williams. 2010. Information in support of indicator selection for monitoring the Tarium Niryutait Marine Protected Area (TNMPA). Fisheries and Oceans Canada Canadian Technical Report of Fisheries and Aquatic Sciences No. 094.
- Loseto, L.L., Stern, G.A., Gemmill, B., Deibel, D, Connelly, T., Prokopowicz, A., Fortier, L., Ferguson, S.H. 2009. Summer diet of beluga whales inferred by fatty acid analysis of the eastern Beaufort Sea food web. Journal of Experimental Marine Biology and Ecology. 374: 12-18. doi:10.1016/j.jembe.2009.03.015
- Loseto, L.L. and Ross, P.S. 2011. Organic contaminants in marine mammals: concepts in exposure, toxicity, and management. In Environmental Contaminants in Biota: Interpreting Tissue Concentrations. Edited by W.N.Beyer and J.P.Meador. CRC Press, Taylor & Francis Group, Boca Raton, FL pp. 349-375.
- Luque, S.P. and S.H. Ferguson. 2009. Ecosystem regime shifts have not affected growth and survivorship of eastern Beaufort Sea belugas. Oecologia 160: 367-378. DOI 10.1007/s00442-009-1300-6
- Luque, S.P. and S.H. Ferguson. 2010. Age structure, growth, mortality, and density of belugas (Delphinapterus leucas) in the Canadian Arctic: responses to environment? Polar Biology 33: 163-178.
- Lynch, M., O. Nielsen, P. J. Duignan, R. Kirckwood, A. Hoskins, and J. P. Y. Arnould. 2011. Serologic Survey For Potential Pathogens and Assessment of Disease Risk in Australian Fur Seals. Journal of Wildlife Diseases 47:555-565.
- Lynch, M., P. J. Duignan, T. Taylor, O. Nielsen, R. Kirckwood, J. Gibbons, and J. P. Y. Arnould. 2011. Epizootiology of Brucella Infection in Australian Fur Seals. Journal of Wildlife Diseases 47:352-363.
- Marcoux, M, M Auger-Méthé, E Chmelnitsky, SH Ferguson, MM Humphries. 2011. Local passive acoustic monitoring of narwhals in the Canadian Arctic: A pilot project. Arctic 64: 307-316.
- Marshall, H. D., K. A. Hart, E. S. Yaskowiak, G. B. Stenson, D. McKinnon and E. A. Perry. 2010. Molecular identification of prey in the stomach contents of harp seals (Pagophilus groenlandicus) using species-specific oligonucleotides. Mol. Ecol. Res. 10:181-189.
- Matthews, CJD, SP Luque, SD Petersen, RD Andrews, SH Ferguson. 2011. Satellite tracking of a killer whale (Orcinus orca) in the eastern Canadian Arctic documents ice avoidance and rapid, long-distance movement into the North Atlantic. Polar Biology DOI 10.1007/s00300-010-0958-x
- Morissette, L. and M.O. Hammill. 2011. A preliminary evaluation of the impacts of grey seal, Halichoerus grypus, predation on the 4T ecosystem and possible effects of their removal on cod (Gadus morhua) recovery. CSAS Res Doc. 2011/016
- Morissette, L., M. Castonguay, C. Savenkoff, D. P. Swain, D. Chabot, H. Bourdages, M. O. Hammill and J. M. Hanson. 2009. Contrasting changes between the northern and southern Gulf of St. Lawrence ecosystems associated with the collapse of groundfish stocks. Econorth Proceedings in DSRII. Deep–Sea Research II 56:2117-2131.
- Mos, L., Cameron, M., Jeffries, S.J., Koop, B.F., and Ross, P.S. 2010. Risk-based analysis of PCB toxicity in harbor seals. Integrated Environmental Assessment and Management 6: 631-640.
- Mosnier, A., Lesage, V., Gosselin, J.-F., Lemieux Lefebvre, S., Hammill, M. O., Doniol-Valcroze, T. 2009. Information relevant to the documentation of habitat use by St. Lawrence beluga (Delphinapterus leucas), and quantification of habitat quality. DFO CSAS Research Document 2009/098.
- Mouy, X., Bahoura, M., and Simard, Y. 2009. Automatic recognition of fin and blue whale calls for real-time monitoring in the St. Lawrence. J. Acoust. Soc. Am. 126(6): 2918-2928.
- MPO, 2011. Ajout d’un brise-lame au quai des pilotes des Escoumins, Québec – effets potentiels sur les mammifères marins. Secrétariat canadien de consultation scientifique du MPO, Réponse des Sciences. 2011/007. 11 pp
- MPO, 2011. Réfection des embarcadères de Tadoussac et Baie-Sainte-Catherine, Québec – effets sur les mammifères marins. Secrétariat canadien de consultation scientifique du MPO, Réponse des Sciences. 2011/009. 13 pp.
- Nichol, L.M. 2009. An assessment of some of the factors affecting observer efficacy during cetacean surveys in Canada’s Pacific Region. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/065. iv + 18 p.
- Nichol, L.M., R. Abernethy, L. Flostrand, T.S. Lee and J.K.B. Ford. 2010. Information relevant for the identification of Critical Habitats of North Pacific Humpback Whales (Megaptera novaeangliae) in British Columbia. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/116. iv + 40p.
- Nichol, L.M., Boogaards, M.D., Abernethy, R. 2009. Recent trends in the abundance and distribution of sea otters (Enhydra lutris) in British Columbia. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/016. iv + 16 p.
- Noël, M., Barrett-Lennard, L.G., Guinet, C., Dangerfield, N., and Ross, P.S. 2009. Persistent Organic Pollutants (POPs) in killer whales (Orcinus orca) from the Crozet Archipelago, southern Indian Ocean. Mar.Environ.Res. 68: 196-202.
- Noël, M., Dangerfield, N., Hourston, R.A.S., Belzer, W., Shaw, P., Yunker, M.B., and Ross, P.S. 2009. Do trans-Pacific air masses deliver PBDEs to coastal British Columbia, Canada? Environ.Pollut. 157: 3404-3412.
- Norman, S.A., S. Raverty, E. Zabek, S. Etheridge, J.K.B. Ford, L.M.N. Hoang, and M. Morshed. 2011. Maternal-fetal transmission of Cryptococcus gattii in harbor porpoise. Emerging Infectious Diseases 17: 304-305.
- Nweeia , M. T., Thackeray, J. F., Eichmiller, F., Richard, P, Leclerc, L-M., Lanham, J. and I. Newton. 2009. A note on isotopic analysis of sectioned tusks of the narwhal (Monodon monoceros) and tusk growth rates. Annals of the Transvaal Museum 45: 138-142.
- Olesiuk, P.F., Lawson, J.W., and Trippel, E.A. 2010. Pathways of effects of noise associated with aquaculture on natural marine ecosystems in Canada, DFO Can. Sci. Advis. Sec. Res. Doc., Ottawa, ON. 2010/025.
- Palacios, G., J. F. X. Wellehan, Jr, S. Raverty, A. V. Bussetti, J. Hui, N. Savji, H. H. Nollens, D. Lambourn, C. Celone, S. Hutchison, C. H. Calisher, O. Nielsen and W. I. Lipkin. 2011. Discovery of an orthoreovirus in the aborted fetus of a Steller sea lion (Eumetopias jubatus) Journal of General Virology, 92, 2558–2565.
- Parsons, K.M., Balcomb, K.C. III, Ford, J.K.B., and Durban, J.W. 2009. The social dynamics of southern resident killer whales and conservation implications for this endangered population. Animal Behaviour 77:963-971
- Paulic, J.E., B. Bartzen, R. Bennett, K. Conlan, L. Harwood, K. Howland, V. Kostylev, L. Loseto, A. Majewski, H. Melling, A. Neimi, J.R. Reist, P. Richard, E. Richardson, S. Solomon, W. Walkusz and B. Williams. 2012. Ecosystem Overview Report for the Darnley Bay Area of Interest (AOI). DFO Can. Sci. Advis. Sec. Res. Doc. 2011/062. vi + 63 p.
- Petersen, S.D., M.H.Hainstock and P.J. Wilson. 2010. Population genetics of Hudson Baymarinemammals: current knowledge and future risks. In: S.H.Ferguson, L.L. Loseto, and M.Mallory (eds.) Global Warming and Arctic Marine Mammals. Springer, New York. pp 237-267
- Petersen, S.D., Tenkula, D., and Ferguson, S.H. 2011. Population Genetic Structure of Narwhal (Monodon monoceros).DFO Can.Sci.Advis.Sec.Res.Doc.2011/021.vi + 20 p.
- Petersen, S.D., Tenkula, D., Ferguson, S.H., Kelley, T., and Yurkowski, D.J. 2012. Sex determination of belugas and narwhals: understanding implications of harvest sex ratio. DFO Can. Sci. Advis. Sec. Res. Doc. 2012/019. iii + 9 p.
- Petersen, S.D., Tenkula, D. and Ferguson, S.H. 2011. Population Genetic Structure of Narwhal (Monodon monoceros). DFO Can. Sci. Advis. Sec. Res. Doc. 2011/021. vi + 20 p.
- Petersen, S.E., S.H.Ferguson.2010. Advice regarding the genetic structure of Canadian narwhal (Monodon monoceros). DFO Can. Sci. Advis. Sec. Sci. Advis. Rep.2011/021.
- Piché, C., L. Measures, C. Bédard and S. Lair. 2010. Bronchoalveolar lavage and pulmonary histopathology in harp seals (Phoca groenlandica) experimentally infected with Otostrongylus circumlitus. Journal of Wildlife Diseases 46: 409-421.
- Pomerleau, C., S.H. Ferguson, W. Walkus. 2011. Stomach contents of bowhead whales (Balaena mysticeus) from four locations in the Canadian Arctic. Polar Biology 34(4):615-620.
- Pomerleau, C., Ferguson, S.H., Luque, S., Patterson, T.A., Lesage, V., Heide-Jørgensen, M.-P., Dueck, L.L. 2011. Bowhead whale Balaena mysticetus diving and movement patterns in the Eastern Canadian Arctic: implications for foraging ecology. Endangered Species Research 15:167-177.
- Pomerleau, C., Winkler, G., Sastri, A., Nelson, J., Vagle, S., Lesage, V., Ferguson, S. 2011. Spatial patterns in zooplankton communities across the eastern Canadian subarctic and Arctic waters: Insights from stable carbon (δ13C) and nitrogen (δ15N) isotope ratios. Journal of Plankton Research. 33(12):1779-1792.
- Prewitt, J.S., Freistroffer, D.V., Schreer, J.F., Hammill, M.O., Burns, J.M. 2010. Postnatal development of muscle biochemistry in nursing harbor seal (Phoca vitulina) pups: Limitations to diving behavior? Journal of Comparative Physiology – B. 180:757–766
- Raach, M., Lebeuf, M. et Pelletier, É. (2011) PBDEs and PCBs in the liver of the St. Lawrence Estuary beluga (Delphinapterus leucas) : A comparison of levels and temporal trends with the blubber. J. Environ. Monit., 13 : 649-656.
- Richard , P.R., Laake, J.L. Hobbs , R.C., Heide-Jørgens en, M.P., Asselin, N.C. and Cleator H. 2010. Baffin Bay Narwhal Population Distribution and Numbers: Aerial Surveys in the Canadian High Arctic, 2002-04. Arctic 63(1): 85-99
- Richard, P. 2009. Marine Mammals of Nunavut. Qikiqtani School Operations, Dept. of Education, Nunavut. 97 p. (2nd reprint with corrections).
- Richard, P.R. 2010. Stock definition of belugas and narwhals in Nunavut. CSAS Research Document - 2010/022: 18 p.
- Richard, P.R. 2010. Survey Index of the Northern Hudson Bay Narwhals, August 2008. CSAS Research Document 2010/021: 21 p.
- Richard, P.R. 2011. Allocation model for landed catches from Baffin Bay narwhal stocks. CSAS Research Document 2011/056: 31 p.
- Ross, P. S. 2010. Impact of at sea disposal on resident killer whale (Orcinus orca) Critical Habitat: Science in support of risk management. Fisheries and Oceans Canada Science Advisory Secretariat: Science Advisory Report No. 046. 1-9 pp.
- Ross, P.S., Couillard, C.M., Ikonomou, M.G., Johannessen, S.C., Lebeuf, M., Macdonald, R.W. and Tomy, G.T. 2009. Large and growing environmental reservoirs of Deca-BDE present an emerging health risk for fish and marine mammals. Marine Pollution Bulletin 58:7-10.
- Ross, P.S., Barlow, J., Jefferson, T.A., Hickie, B.E., Lee, T., MacFarquhar, C., Parsons, E.C.M., Riehl, K., Rose, N., Simpkins, M., Slooten, E., Tsai, J., Wang, J.Y., Wright, A., and Yang, S.-C. 2011. Ten guiding principles for the delineation of priority habitat for coastal cetaceans. Marine Policy 35: 483-488.
- Ross, P.S., Dungan, S.Z., Hung, S.K., Jefferson, T.A., MacFarquhar, C., Perrin, W.F., Riehl, K., Slooten, E., Wang, J.Y., White, B.N., Wursig, B., Yang, S.-C., and Reeves, R.R. 2010. Averting the Baiji syndrome: Characterising habitat for critically endangered dolphins in eastern Taiwan Strait. Aquatic Conservation: Marine and Freshwater Ecosystems 20: 685-694.
- Roux, M.-J., Harwood, L. A., Illasiak, Babaluk, J., and de Graff, N. In press. Fishery resources and habitats in a headwater lake of the Brock River, NT, 2003-2005. Can. Manuscript Report Fisheries and Aquatic Sciences 2932.
- Roy, N., Simard, Y., and Gervaise, C. 2010. 3D tracking of foraging belugas from their clicks: Experiment from a coastal hydrophone array. Applied Acoustics 71: 1050-1056. DOI: 10.1016/j.apacoust.2010.05.008
- Salberg, A-B., T. A. Øigard, G. B. Stenson, T. Haug and K. T. Nilssen. 2009. Estimation of seal pup production from aerial surveys using generalized additive models. Can. J. Fish. Aquat. Sci. 66:847-858.
- Sandstrom, S., Harwood, L. A., and Howland, K. Status of anadromous 2009. Status of anadromous Dolly Varden (Salvelinus malma) of the Rat River, Northwest Territories, as assessed through sampling of the subsistence fishery (1995-2007). Canadian Manuscript Report of Fisheries and Aquatic Sciences 2891.
- Savenkoff, C. Valois, S., Chabot, D. and Hammill, M.O. 2009. Input data and parameter estimates for ecosystem models of the northern Gulf of St. Lawrence. (2003-2005). Can. Tech. Rep. Fish. Aquat. Sci. 2829. 117 p.
- Scheer, J.F., J. L. Lapierre, & M. O. Hammill. 2010. Stomach Temperature Telemetry Reveals that Harbor Seal (Phoca vitulina) Pups Primarily Nurse in the Water. Aquatic Mammals 36:270-277.
- Séquin, G., F. Bouchard, L.N. Measures, C.F. Uhland, and S. Lair. 2011. Infections with Philometra sp. associated with mortalities in wild-hatched captive-raised striped bass, Morone saxatilis (Walbaum). Journal of Fish Pathology 34: 475-481.
- Shelley, L.K., Balfry, S.K., Ross, P.S., and Kennedy, C.J. 2009. Immunotoxicological effects of a sub-chronic exposure to selected current-use pesticides in rainbow trout (Oncorhyncus mykiss). Aquat.Toxicol. 92: 95-103.
- Simard, Y. 2009. Le parc marin du Saguenay–Saint-Laurent: un habitat exceptionnel pour les baleines. Nat. Can. 133(3) : 57-61.
- Simard, Y. 2009. Le Parc Marin Saguenay–Saint-Laurent: processus océanographiques à la base de ce site d’alimentation unique des baleines du Nord-Ouest Atlantique. The Saguenay–St. Lawrence Marine Park: oceanographic process at the basis of this unique forage site of Northwest Atlantic whales. Rev. Sc. Eau / J. Water Sci. 22(2): 177-197.
- Simard, Y. 2009. Passive acoustic monitoring during seismic surveys / Le monitorage par acoustique passive pendant les relevés sismiques. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/068. iv + 22 p.
- Simard, Y. and Harvey, M. 2010. Predation on northern krill (Meganyctiphanes norvegica Sars). In. G.A. Tarling, ed. Biology of Northern Krill. Adv. in Mar. Biol. Vol. 57: 277-306. (Burlington: Academic Press, ISBN: 978-0-12-381308-4)
- Simard, Y., and Sourisseau, M. 2009. Diel changes in acoustic and catch estimates of krill biomass. ICES J. Mar. Sci. 66: 1318-1325. doi: 10.1093/icesjms/fsp055
- Simard, Y., Lepage, R., and Gervaise, C. 2010. Anthropogenic sound exposure of marine mammals from seaways: Estimates for lower St. Lawrence Seaway, eastern Canada. Applied Acoustics 71: 1093-1098. DOI: 10.1016/j.apacoust.2010.05.012
- Simard, Y., Roy, N., Gervaise, C., Giard, S., Conversano M., and Ménard, N. 2010. Passive acoustics applied to white whale density monitoring and 3D tracking their foraging dives in an ecosystem hot spot. Proc. IEEE OES Passive 2010, Brest, France. 23-24
- Simard, Y., Roy, N., Giard, S., Gervaise, C., Conversano, M., and Ménard, N. 2010. Estimating whale density from their whistling activity: example with St. Lawrence beluga. Applied Acoustics 71: 1081-1086. DOI: 10.1016/j.apacoust.2010.05.013
- Sjare B., and Stenson, G.B. 2010 .Changes in the reproductive parameters of female harp seals (Pagophilus groenlandicus) in the Northwest Atlantic. ICES Journal of Marine Science 67:304 -315.
- Spaven, L.D., Ford, J.K.B., and C. Sbrocchi. 2009. Occurrence of leatherback sea turtles off the Pacific coast of Canada, 1931-2009. Can. Tech. Rep. Fish. Aquat. Sci. 2858: 32 p.
- Stenson G. B. 2009. Total removals of Northwest Atlantic harp seals (Pagophilus groenlandicus), 1952-2009. CSAS Res. Doc. 2009/112
- Stenson, G.B., M. O. Hammill and J. Flight.2010. Winter diet of grey seals in Cabot Strait. CSAS Res Doc. 2010/128
- Stenson, G. B., M. O. Hammill and B. Healey. Estimating Reproductive Rates of Northwest Atlantic Harp Seals, 1954-2007, for Population Modelling CSAS Res. Doc. 2009/113
- Stenson, G.B. and M.O. Hammill 2010. Improving the management of Atlantic seals under the precautionary approach. Canadian Science Advisory Secretariat Res. Doc. 2010/135.
- Stenson, G.B. and N. J. Wells. 2011 Current reproductive and maturity rates of Northwest Atlantic harp seals, Pagophilus groenlandicus. Canadian Science Advisory Secretariat Res. Doc. 2010/136.
- Stenson, G.B., M.O. Hammill and B. Healey. 2009. Reproductive rates of Northwest Atlantic harp seals, 1954-2007. CSAS Res. Doc 2009/113.
- Stenson, G.B., Hammill, M.O., and Lawson, J.W. 2009. Estimating pup production of Northwest Atlantic Harp Seals, Pagophilus groenlandicus: Results of the 2008 surveys, DFO Can. Sci. Advis. Sec. Res. Doc., Ottawa, ON. 2009/103. iv + 39p.
- Stenson, G.B., Hammill, M.O., and Lawson, J.W. 2011. How many harp seal pups are there? Additional results from the 2008 surveys 2010/137, DFO Can. Sci. Advis. Sec. Res. Doc., Ottawa, ON. 2010/137. iv + 19p.
- Stenson, G.B., S. Benjamins and D.G. Reddin. 2011 Using bycatch data to understand habitat use of small cetaceans: lessons from an experimental driftnet fishery. ICES J. Mar. Sci. 68:937-946.
- Stevens, T.S., and Lawson, J.W. 2009. Passive acoustic monitoring (PAM) sound catalogue: Approach for NW Atlantic additions, Report to Environmental Studies Research Funds, National Energy Board, Calgary, AB. 28p.
- Stewart, R. E. A. and B. E. Stewart, 2009. Female reproductive systems. Pages 423-428 in Encyclopedia of Marine Mammals, Second Edition. W. F. Perrin, B. Würsig and J. G. M. Thewissen (editors). Academic Press, San Diego. 1316 pp
- Stewart, R.E.A. 2010 Walrus. The Canadian Encyclopedia, The Historica-Dominion Institute, Toronto, http://thecanadianencyclopedia.com April 2010
- Stewart, R.E.A., V. Lesage, J.W. Lawson, H. Cleator and K.A. Martin. 2012. Science Technical Review of the draft Environmental Impact Statement (EIS) for Baffinland’s Mary River Project. DFO Can. Sci. Advis. Sec. Res. Doc. 2011/086. vi + 62 p.Res Doc – submitted Oct 2011
- Stokesbury, M.J.S., Dadswell, M.J., Holland, K.N., Jackson, G.D., Bowen, W.D.and R.K.O’Dor. 2009. Tracking diadromous fishes at sea using hybrid acoustic and archival tags. In: Haro A., Smith K.L., Rulifson R.A., Moffitt C.M., Klauda R.J., et al, (eds). Challenges for Diadromous Fishes in a Dynamic Global Environment. Bethesda: American Fisheries Society Symposium 69.
- Straneo, F., Hamilton, G.S., Sutherland, D.A., Stearns, L.A., Davidson, L., Hammil, M.O., Stenson, G.B. and Rosing-Asvid, A. 2010. Rapid circulation of warm subtropical waters in a major glacial fjord off East Greenland. Nature Geoscience 3:182-186
- Swain, D.P., H.P. Benoit, and M.O. Hammill. 2011. Grey seal reduction scenarios to restore the southern Gulf of St. Lawrence cod population. CSAS Res Doc. 2011/035
- Swain, D. P., Benoît, H. P., Hammill, M. O., McClelland, G., and Aubry, É. 2011. Alternative hypotheses for causes of the elevated natural mortality of cod (Gadus morhua) in the southern Gulf of St. Lawrence: the weight of evidence. DFO Can. Sci. Advis. Sec. Res. Doc. 2011/036. iv + 33 p.
- Templeman, N.D., Campana, S., Colbourne, E., Pepin, P., Power, D., Kulka, D.W., Lawson, J.W., Murphy, E., Simpson, M.R., Miri, C.M., Koen-Alonso, M., Ollerhead, L.M.N., Healey, B., and Wareham, V. 2010. Biophysical Overview of the Laurentian Channel Area of Interest (AOI). DFO Can. Sci. Advis. Sec. Sci. Advis. Rep. 2010/076.
- Thomas, L., M.O. Hammill and W.D. Bowen. 2011. Assessment of population consequences of harvest strategies for the northwest Atlantic grey seal population. CSAS Res Doc. 2011/007
- Thomas, L., M.O. Hammill and W.D. Bowen. 2011. Estimated size of the Northwest Atlantic grey seal population 1977-2010. CSAS 2011/017
- Tierney, K.B., Baldwin, D.H., Hara, T.J., Ross, P.S., Scholz, N.L., and Kennedy, C.J. 2010. Review: Olfactory toxicity in fishes. Aquat. Toxicol. 96: 2-26.
- Tollit, D., Pierce, G.J., Hobson, K. A., Bowen, W. D., and Iverson, S. J. 2010. Diet, p. 165-190. In Boyd, I. L., Bowen, W. D., and Iverson, S. J. (eds.) Marine Mammal Ecology and Conservation: A handbook of techniques, Oxford University Press.
- Tomy G.T., K. Pleskach, S.H. Ferguson, J. Hare, G. Stern, G. MacInnis, C.H. Marvin and L. Loseto. 2009. Trophodynamics of some PFCs and BFRs in a western Canadian Arctic marine food web. Environmental Science and Technology 43:4076-4081. DOI: 10.1021/es9
- Treble, M.A. and R.E.A. Stewart. 2009. Impacts and risks associated with a Greenland Halibut (Reinhardtius hippoglossoides) gillnet fishery in inshore areas of NAFO Subarea 0. DFO Can. Sci. Advis. Sec. Res. Doc. 2010/032. vi + 18 p.
- Trzcinski, M. K., Mohn, R. and Bowen, W. D. 2009. Estimating the impact of grey seals on the Eastern Scotian Shelf and Western Scotian Shelf cod populations. DFO Can. Sci. Advis. Sec. Res. Doc. 2009/052.
- Trzcinski, M.K., R. Mohn, and W.D. Bowen. 2011. Estimating the Impact of Grey Seals on the Eastern Scotian Shelf Cod Population. DFO Can. Sci. Advis. Sec. Res. Doc. 2011/078: vi + 20p
- Tucker, S., W. D. Bowen, S. J. Iverson and G. B. Stenson. 2009. Intrinsic and extrinsic sources of variation in the diets of harp and hooded seals revealed by fatty acid profiles. Can. J. Zool. 87:139-151.
- Tucker, S., W. D. Bowen, and S. J. Iverson, W. Blanchard, and G. Stenson. 2009. Sources of variation in diets of harp (Pagophilus groenlandicus) and hooded (Cystophora cristata) seals estimated from quantitative fatty acid signature analysis (QFASA). Marine Ecology Progress Series, 384: 287-302.
- Turgeon, J. , P. Duchesne, L.D. Postma, and M.O. Hammill. 2009. Spatiotemporal distribution of beluga stocks (Delphinapterus leucas) in and around Hudson Bay: Genetic mixture analysis based on mtDNA haplotypes – CSAS Res. Doc. 2009/011
- Van Opzeeland, I.C., P.J. Corkeron, D. Risch, G. Stenson, S.M. Van Parijs. 2009. Geographic Variation in vocalizations of pups and mother-pup behavior of harp seals Pagophilus groenlandicus. Aquatic Biology 6: 109-120
- Vazquez-Medina, J.P., N.O. Olguın-Monroy, P.D. Maldonado, A. Santamarıa, M. Ko¨ nigsberg, R. Elsner, M.O. Hammill, J.M. Burns, and T. Zenteno-Savın. 2011. Maturation increases superoxide radical production without increasing oxidative damage in the skeletal muscle of hooded seals (Cystophora cristata). Canadian Journal of Zoology, 89:(3) 206-212, 10.1139/Z10-107
- Ward, E.J., Parsons, K., Holmes, E.E., Balcomb, K.C. III, and Ford, J.K.B. 2009. The role of menopause and reproductive senescence in a long-lived social mammal. Frontiers in Zoology, 6:4.
- Watt, C.A. and Ferguson, S.H. 2011. Stable isotope and fatty acid analyses of samples from entrapped narwhals (Monodon monoceros). DFO Can. Sci. Advis. Sec. Res. Doc. 2010/134. iv + 12 p.
- Westdal, K. Richard, P. and J. Orr. 2009. Migration route and seasonal home range of the Northern Hudson Bay narwhal (Monodon monoceros). In: Ferguson, SH, Loseto, LL, and ML Mallory. A little less Arctic: top predators in the world’s largest northern inland sea, Hudson Bay. Springer Publishing Company.
- Wiig, Ø., M.P. Heide-Jørgensen, C. Lindqvist, K.L. Laidre, L.D. Postma, L.P. Dueck and L. Bachman. 2011. Recaptures of genotyped bowhead whales (Balaena mysticetus) in Eastern Canada and West Greenland. Endangered Species Research 14:235-242.
- Wood, S.A., T.R. Frasier, B.A. McLeod, J.R. Gilbert, B.N. White, W.D. Bowen, M.O. Hammill, G.T. Waring, and S. Brault. 2011. The genetics of recolonization: an analysis of the stock structure of grey seals (Halichoerus grypus) in the northwest Atlantic. Can. J. Zool. 89: 490–497.
- Young, B.G., L.L. Loseto, and S.H. Ferguson. 2010. Diet differences among age classes of Arctic seals: evidence from stable isotope and mercury biomarkers. Polar Biology 33: 153-162
- Young, BG, JW Higdon, SH Ferguson. 2011. Killer whale (Orcinus orca) photo-identification in the eastern Canadian Arctic. Polar Research 30: online 1-11.
- Yunker, M.B., Lachmuth, C.L., Cretney, W.J., Fowler, B.R., Dangerfield, N.J., White, L., and Ross, P.S. 2011. Biota – sediment partitioning of aluminum smelter related PAHs and pulp mill related diterpenes by intertidal clams at Kitimat, British Columbia. Mar. Environ. Res. 72: 105-126.
- Yurkowski, D.J., M. Chambellant and S.H. Ferguson. 2011. Bacular and testicular growth and allometry in the aquatic-mating ringed seal (Pusa hispida): evidence of polygyny? Journal of Mammalogy 92:803-810.
- Date modified: