Changing ocean chemistry
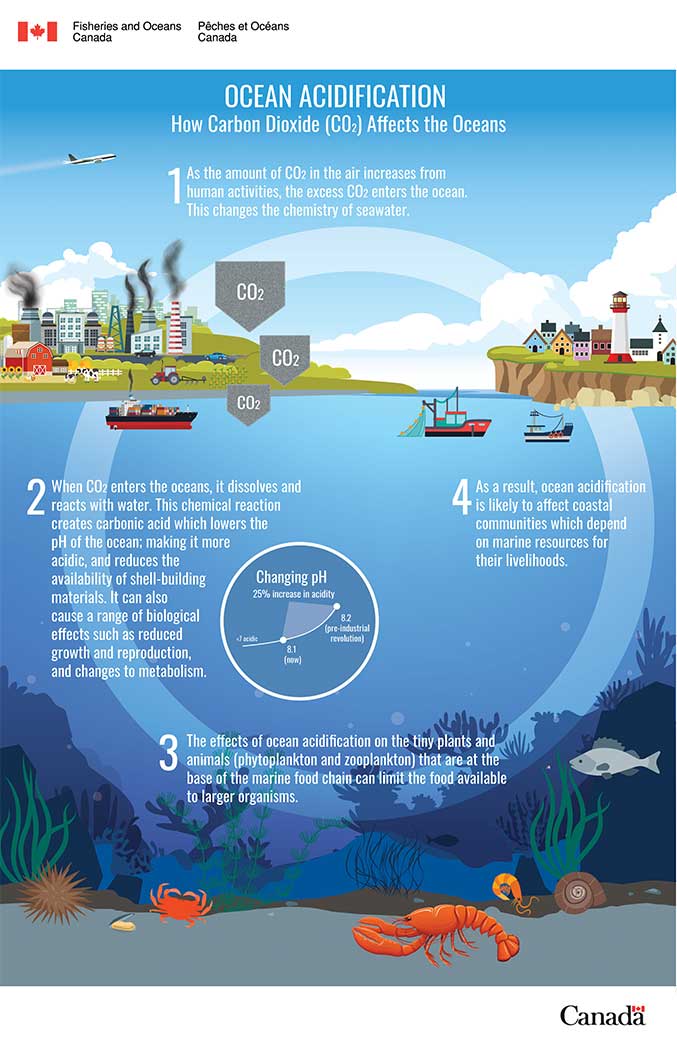
Understand how ocean acidification can make it difficult for many species to survive and thrive. Photo credit: Systemscope infographic.
The changing ocean environment is a critical global issue which threatens the sustainable use of the earth’s ocean by future generations. It is expected that Canada’s oceans will become warmer, fresher, more acidic and less oxygenated (below the surface) as a result of the increasing Carbon Dioxide (CO2) in the atmosphere and the changing climate.
On this page:
Ocean acidification
Ocean acidification is the term that we use to describe the long-term change of ocean chemistry as CO2 is absorbed from the atmosphere. With the increase in human activities producing CO2 since the Industrial Revolution, the world’s oceans are absorbing CO2 more than in the past.
When CO2 is absorbed in the ocean, it reacts with seawater to form a weak acid, called carbonic acid (H2CO3). The carbonic acid (H2CO3) then further breaks down or dissociates into hydrogen (H+), bicarbonate (HCO3-) and carbonate ions (CO32-).
CO2 (aq) + H20 H2CO3 HCO3 + H+ CO32- + 2H+
The results of these chemical reactions include an increase in the amount of hydrogen (H+) ions which is what decreases the pH of the ocean, making it more acidic. There is also a decrease in the availability of carbonate ions (a building block for skeletons and shells of many marine organisms).
The impacts of ocean acidification can be temporally and spatially more variable in coastal areas where other inputs may further contribute to reduction in pH. For example, this includes inputs from oceanographic physical processes such as upwelling, river discharge, nutrient run-off and sewage. These inputs can further lower pH and therefore create regions of even lower pH than the global baseline due to increasing CO2 alone.
A pH scale is how we measure acidity and it indicates how much acid is in a liquid. The scale ranges from 0 to 14 with pH measurements greater than 7 being basic or alkaline, measurements less than 7 are acidic and measurements equal to 7 are neutral. The pH of the ocean is currently slightly basic and will likely never become acidic with ocean acidification, however it is changing in the direction of becoming more acidic (similar to temperatures warming from -20 to 0, still cold but warmer than before). Since the beginning of the Industrial Revolution, the pH of the surface ocean has fallen by 0.1 pH units. While this may not sound like a big change, it is significant because it represents approximately a 30% increase in hydrogen ion concentration. Problems arise when the pH of the ocean changes more quickly than organisms are able to adapt.
It is predicted that the oceans will continue to absorb carbon dioxide and continue to become more acidic. This means that oceans may likely become unsuitable for many species lower in the food chain, and some commercially harvested organisms, including shellfish species from Canadian waters. This will continue for decades after global CO2 emissions in the atmosphere are controlled.
Drivers of ocean acidification in Canada’s three oceans
Canada’s three oceans are particularly vulnerable to the impacts of ocean acidification because CO2 is more readily absorbed in colder waters. In colder waters, the solubility of calcium carbonate shells increases making them more susceptible to the corrosive effects of ocean acidification.
Other factors may also affect the extent and rate at which ocean acidification occurs within a region and there are distinct drivers in each of Canada’s three oceans.
Pacific Region
Pacific Region
Canada’s Pacific Ocean is subject to seasonal upwelling events in coastal areas caused by strong winds which bring deep, cold ocean waters to the surface. This water is generally nutrient rich, has a lower pH and higher CO2 levels than surface ocean layers. These nutrient rich areas are known as productivity ‘hotspots’ and are therefore biologically important. However, in combination with the additional anthropogenic CO2, pH levels continue to decline to critical levels and further intensify the impacts of ocean acidification.
Arctic Region
Arctic Region
The Arctic is one of the world’s most vulnerable areas to ocean acidification. Cold Arctic waters which absorb CO2 more easily from the atmosphere in combination with seasonally variable freshwater inputs from ice-melt, run-off from rivers, and mixing of corrosive waters from the Pacific all contribute to lowering the pH and enhancing the acidification of Arctic waters. Additionally as ice continues to decline in the Arctic, there will be more open water resulting in increasing uptake of atmospheric CO2 which will further accelerate acidification.
Atlantic Region
Atlantic Region
In Canada’s diverse Atlantic Region, a combination of physical ocean processes contribute to increasing acidification. Low oxygen bottom water in the Gulf of the St. Lawrence is originated from the Atlantic and its pH is decreasing much faster than the global average. Freshwater input from the St. Lawrence and other rivers, sea ice melt and Arctic waters (more acidic than the Atlantic water) increases existing ocean acidification in the region. The Labrador Sea in the North Atlantic also enhances ocean acidification because of high rates of CO2 uptake due to deep convection in winter.
Hypoxia
Changing global and regional climates also make marine ecosystems more vulnerable to reduced oxygen levels (hypoxia). Aquatic organisms need dissolved oxygen to survive under water. In a warming climate, surface water warms faster, and becomes lighter than the waters that are beneath them. Mixing different depths of water helps provide oxygen to deeper water. It is harder to mix lighter, warmer surface water with denser bottom water. Reduced mixing means that some areas won’t have as much oxygen. At oxygen levels below 30%, species that cannot tolerate low oxygen conditions must migrate to other geographic regions. If they can’t migrate, their survival may be at risk.
Fish, shellfish and crustaceans depend on dissolved oxygen in seawater to survive. Decreasing oxygen levels – or hypoxia – can cause serious problems for these species, as well as many others.
Oxygen levels are decreasing in nearly all marine waters surrounding Canada. The highest rates of decline are in the Gulf of St. Lawrence, Hudson Bay and the Gulf of Alaska. Some shallow waters, such as the estuaries in Prince Edward Island and New Brunswick, experience short-term hypoxia that only lasts a few hours or days. Some fjords, such as the Bedford Basin in Nova Scotia, and coastal inlets, such as Saanich in British Columbia, become hypoxic or anoxic (a severe form of hypoxia) during certain seasons. In northern Canada, oxygen levels may be decreasing in the Beaufort Sea.
It is likely that global warming-related changes in ocean currents and ocean mixing likely play a role in these oxygen declines.
Ongoing research
Our scientists conduct monitoring of ocean acidification and other chemical changes to assess the state of coastal and offshore waters. By studying trends and variability in key climate indicators over decades, we can better understand and predict the future state of Canada’s oceans.
This work is designed specifically to:
- help us understand the interaction of ocean acidification with other climate stressors and changing ocean conditions such as hypoxia, temperature, ocean circulation, or freshwater inputs
- project and predict future climate and ocean conditions such as water temperature, currents and ocean chemistry
Additionally, we work with partners in Canada, including academia, non-profit groups, industry and stakeholder groups, and international partners such as the United States, Asia, and EU countries, to coordinate current and future ocean acidification observing and monitoring activities.
The changing ocean environment is a critical global issue which threatens the sustainable use of the earth’s ocean by future generations. Marine ecosystems in Canada are undergoing changes which are related to a combination of climate change, natural variability, and human activities. It is expected that Canada’s oceans will become warmer, fresher, more acidic and less oxygenated (below the surface) as a result of the increasing Carbon Dioxide (CO2) in the atmosphere. The potential impacts these changing ocean conditions are creating a sustained need for scientific expertise on what the impacts will be for Canada’s oceans.
Access our completed research studies and ongoing monitoring activities.
Access the Issue Paper: Ocean Acidification in Canada’s Coastal Waters, developed by Canadian Climate Forum in Partnership with DFO.
Access our Collaborative Framework: A Collaborative Framework for Joint DFO/NOAA Ocean Acidification Research and Monitoring, developed in Partnership with the US National Oceanic and Atmospheric Administration.
- Date modified:


