Geoduck clam (Panopea generosa): Anatomy, Histology, Development, Pathology, Parasites and Symbionts
'Wart' Transmission Experiments
To date, no obvious pathogenic agent has been observed in conjunction with ‘warts' in geoduck clams. Histological observations indicate that the ‘warts’ which contain packed dead and dying cells are the result of a haemocytic reaction in the musculature of the siphon or the mantle causing a concentration of extremely high numbers of haemocytes. Apparently, the lesion (wart) moves in some, as yet unexplained manner, from the interior tissues to the surface, where it is eventually walled off from the underlying tissues with the periostracum. Thus, the ‘wart’ may be part of a healing mechanism of the geoduck clam. In order to determine whether ‘warts’ or pustules and lesions are caused by an infectious organism, three experiments of exposing unaffected cultured juvenile geoduck clams to material collected from ‘warts’ were conducted. In all experiments, cultured juvenile geoduck clams were obtained from grow-out sites on the east coast of Vancouver Island, maintained in the laboratory in ambient flow through seawater and fed approximately 4 litres of a combination of cultured algae [two flagellates (Isochrysis galbana and Pavlova lutheri) and a diatom (Thalassiosira pseudonana)] five times per week. In order to prevent the introduction of potential infectious disease agents from these clams into the local environment, all effluent was disinfected with chlorine prior to discharge to the Nanaimo city sewage system.
Experiment 1
‘Warts’ were excised from three adult geoduck clams collected from Yellowbank on the west coast of Vancouver Island (Fisheries Management Area 24) in October 1996. Excised material included fifteen ‘old’ 'warts' from the surface and three subsurface, purulent lesions. A solution was produced by blending the 'warts' and lesions in sterile filtered sea water, then filtering the resultant mixture through successively smaller nytex screens to 60 µm. A total volume of about 55 ml of ‘wart’ substance in solution was produced; 3.5 ml was utilised as an inoculum and the remainder was added to approximately 3 litres of sea water to act as a slurry for exposure by immersion.
On November 5, 1996, four groups (A-D) of 25 juvenile (95 year class) geoduck clams per group were selected. Group A experienced no experimental procedures to serve as an untreated control. Each juvenile geoduck clam in Group B was injected with 0.05 ml sterile, filtered sea water into the musculature of the right ventral surface of the siphon and the muscular mantle to serve as inoculation control. Geoduck clams in Group C were inoculated with 0.05 ml solution of wart solution, described above, in the same locations as in group B. Geoduck clams in Group D were immersed in the slurry with an airstone and maintained overnight in a 3 liter container surrounded by cold (about 10°C), flowing seawater. The results of this experiment is presented in Table 1.
Table 1: The number of geoduck clams exhibiting induced ‘warts’ on various dates after initiation of the experiment.
| Date
1996 to 1997 |
Group A
negative control (n=25) |
Group B
sterile injection (n=25) |
Group C
wart inoculation n=25) |
Group D
slurry immersion (n=25) |
|---|---|---|---|---|
| Nov. 8 | 0 | 0a | 14 | 0 |
| Nov.12 | 0 | 0a | 18 | 0 |
| Nov.22 | 0 | 0 | 17b | 0 |
| Dec.5 | 0 | 0 | 15c | 0 |
| Dec. 20 | 0 | 0 | 13c | 0 |
| Jan. 13 | 0 | 0 | 11d | 0 |
| Jan. 29 | 0 | 0 | 9c | 0 |
| Feb. 5 | 0 | 0 | 9 | 0 |
| Feb. 20 | 0 | 0 | 9 | 0 |
| March 10 | 0 | 0 | 7d | 0 |
- an inflammatory reaction to the injections was observed in seven geoduck clams
- one mortality
- one mortality and one healed
- two healed

Figure 1. Two juvenile geoduck clams with 'warts' (arrows) on the siphon and mantle where they had been inoculated with 'wart' substance removed from affected wild geoduck clams.
These results appear to indicate that an agent was transmitted by inoculation to induce the formation of ‘warts’ (Fig. 1) and that affected geoduck clams were capable of recovery. In order to confirm these findings and to establish whether the reactions were caused by an organism or simply a reaction to foreign proteins, a second experiment was conducted.
Experiment 2
On December 5, 1996, the ‘warts’ substance from the oozy base of three fused pustules was removed from a single wild adult geoduck collected on November 23, 1996 from near Tofino on the west coast of Vancouver Island (Fisheries Management Area 24). The material, collected solely from subsurface, purulent lesions was mixed with filtered, sterile sea water to make 20 ml of solution, then filtered through a 60 µm nytex screen. This solution was divided to prepare three types of inoculum: 1) untreated, 2) treated with antibiotics of 2000 U/ml each of Penicillin G and Streptomycin, and 3) filtered through a 22 µm Millipore filter to remove all protozoa and bacteria. Four groups (A-D) of juvenile (95 year class) geoduck clams were inoculated with 0.05 ml in two places, as described in experiment 1 (the right ventral surface of the siphon and the muscular mantle). Group A received untreated ‘wart’ solution. Group B received ‘wart’ solution with antibiotics. Group C received ‘wart’ solution filtered to remove protozoa and bacteria. Group D were to serve as controls and were inoculated with heat inactivated bovine serum contaminated with airborne bacteria. The results of this experiment is presented in Table 2. Each of the inocula were plated on TSA and TCBS agar plates (commercial Difco products) and incubated at about 15°C to assay for the presence of viable bacteria.
Table 2. The number of geoduck clams exhibiting induced ‘warts’ on various dates after inoculation. The number of moribund and discarded geoduck clams is indicated in parenthesis.
| Date
1996 to 1997 |
Group A
untreated wart material (n=35) |
Group B
wart with antibiotics (n=35) |
Group C
wart filtered (n=34) |
Group D
bovine serum (n=34) |
|---|---|---|---|---|
| Dec.13 | 0 | 0 | 0 | 0 |
| Dec.16 | 14 | 1 | 11 | 7 (1) |
| Dec.20 | 15 | 1 | 12 (1) | 10 |
| Jan. 3 | 16 (3) | 2 | 14 (1) | 11 |
| Jan. 13 | 17 | 2 | 17 | 8 |
| Jan. 20 | 15 (2) | 1 | 20 | 10 |
| Jan. 29 | 15 | 1 | 15 | 9 (1) |
| Feb. 5 | 14 (1) | 3 (1) | 21 | 11 |
| Feb. 12 | 13 | 2 | 16 (2) | 9 |
| Feb. 20 | 12 | 2 | 17 | 10 (1) |
| Feb. 28 | 11 | 4 | 19 (1) | 12 |
| March 17 | 10 | 2 | 18 | 6 |
Results appear to indicate that ‘wart’ formation is in reaction to a non-specific stimulus which was mitigated to some extent by antibiotics. The histopathology of the induced ‘warts’ was similar to that observed in naturally infected wild geoduck clams. It was interesting to note that no bacteria were cultured from any of the three ‘wart’ inocula preparations but bacteria were present in the bovine serum.
Experiment 3
In March 1998, a third attempt was made to determine if ‘warts’ were caused by an infectious organism and to document the development of the lesions. Healthy, unaffected, cultured, two-year old juvenile geoduck clams (year class 96 that had been planted for one year off Texada Island, fisheries management area 16-21) were obtained for use in this ‘wart’ transmission experiments. The juvenile geoduck clams were divided into four groups designated as A, B, C, and D. All the juveniles were inoculated with either a solution of ‘wart’ material collected from a ‘wart’ or with sterile foetal calf serum (control). Sterile foetal calf serum, a commercially prepared protein solution, was injected (0.05 ml) in the siphon and in the mantle of each of the geoduck clams in groups A and B. The inoculum of ‘wart’ material was prepared by aseptically collecting the subsurface purulent material from one ‘wart’ in the musculature of the siphon of an adult wild geoduck clam sampled from Fisheries Management Area 23-6 (Barkley Sound) in February 1998 (Fig. 2). The material was mixed in a ratio of about 1:10 with sterilised filtered seawater and 0.05 ml was inoculated into the siphon and the mantle of each geoduck in groups C and D. The course of infection was followed in geoduck clams from groups A and C, and at various days post inoculation, the injection sites in geoduck clams from groups B and D were examined histologically.
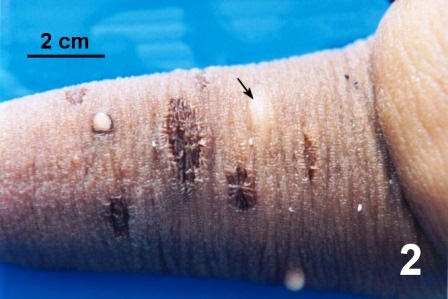
Figure 2. Three 'warts' and several scars on the siphon of a geoduck clam collected from the west coast of Vancouver Island (area 23). The contents of the 'wart' within the musculature (arrow) were used to produce the inoculum for injection in experiment 3.
Groups A and C contained 30 geoduck clams each; the reaction of each clam was monitored over a period of one month. The gross reaction to the inoculation of a foreign protein, calf serum, was very slight compared to the reaction to the injection of ‘wart’ material (Table 3). A dead geoduck clam was found 9 days post inoculation and a total of four geoduck clams inoculated with ‘wart’ material (Group C) died over the thirty day period.
Table 3. The gross effect of inoculation on geoduck clams in Groups A and C over the period of one month.
| Date
1998 |
Days Post Inoculation | Group A
serum inoculation (n=30) |
Group C
‘wart’ inoculation (n=30) |
|---|---|---|---|
| March 5 | 1 | 14 with a slight white mark at the injection site on the mantle | 29 with a slight pink mark at the injection site on the mantle |
| March 9 | 5 | 1 with a slight white mark at the injection site on the mantle | 8 with a pink mark on the siphon site
18 with a pink mark on the mantle site |
| March 11 | 7 | 4 with clear blisters on siphon site | 12 with an orange mark on both the siphon and mantle sites
6 with an orange mark on the siphon 4 with an orange mark on the mantle |
| March 13 | 9 | 2 with a pink wart on the siphon
2 with clear blisters on the siphon |
13 with orange warts on both the siphon and mantle
6 with orange warts on the siphon 1 with an orange wart on the mantle 1 mortality |
| March 20 | 16 | 2 with a pink wart on the siphon
2 with a faint mark on the mantle |
13 with orange warts on both the siphon and mantle
7 with orange warts on the siphon 2 with orange warts on the mantle 1 mortality |
| March 27 | 23 | 1 with an orange wart on the siphon
2 with a faint mark on the mantle |
10 with orange warts on both the siphon and mantle
8 with orange warts on the siphon 2 with faint warts on the mantle 2 with clear blisters covering warts on the mantle 2 mortalities |
| April 3 | 30 | 1 with an orange wart on the siphon
3 with a clear blister on the siphon 5 with a faint mark on the mantle |
13 with orange warts on both the siphon and mantle
5 with orange warts on the siphon 4 with faint warts on the mantle |
On April 22 (day 49) five geoduck clams from Groups A and C were selected for photographic documentation of ‘wart’ appearance. All of the clams inoculated with the calf serum (Group A) were alive and healthy when the observations were terminated on May 6 (day 63). Twenty-five of the thirty geoduck clams inoculated with the ‘wart’ material (Group C) survived until May 6. After May 6, only the five clams from both Groups A and C used for photographic documentation were retained for monitoring photographically until July 8, 125 days post inoculation (Figs. 3 to 8). After 49 days, four of the five Group A geoduck clams (Fig. 3) had a small scar on mantle site and one geoduck clam had a small wart on the siphon. In Group C all the inoculation sites except the mantle site on one clam had a ‘wart’ and/or a blister (Fig. 4). At 84 days post inoculation, two clams in Group A are completely healed and the other three had a slight mark on the mantle (Fig. 5). In Group C one clam had died in late April and was discarded and in the remaining clams the blisters was healing but the ‘warts’ appeared unchanged (Fig. 6). At 125 days post inoculation, the inoculation sites on the mantle of three clams in Group A had only a slight scar (Fig. 7). In Group C, all the lesions in the siphon of the remaining four clams are healed but large scars lingered at the inoculation sites on the mantles (Fig. 8).
| Days after inoculation | Group A
serum inoculation |
Group C
wart inoculation |
|---|---|---|
| Day 49 |  |
 |
| Day 84 |  |
 |
| Day 125 |  |
 |
Groups B (inoculated with foetal calf serum) and D (inoculated with wart material) each consisted of 40 clams. These were sampled for histological examination in groups of three over a period of one month to compare the microscopic reactions to a foreign protein with the microscopic reactions to the ‘wart’ material and to hopefully detect a causative agent or organism in the very early stages of the reaction to ‘wart’ material (Table 4). Specimens were selected for histology on the basis of the most prominent gross reaction; 5 µm sections through the inoculation sites and the viscera were cut and stained with haematoxylin and eosin and examined on a compound microscope at 100 to 1000 times magnification. Group B was not sampled after day 16 as there were no clams with any evidence of a reaction (Table 4).
Table 4. The histological observations of inoculation sites on 15 clams in Group B and on 21 clams in Group D over the period of one month.
| Date 1998 | Days after inoculation | Clam No. | Group B
serum inoculation (n=15) |
Group D
‘wart’ inoculation (n=21) |
|---|---|---|---|---|
| March 5 | 1 | 1
2 3 |
no effect
no effect small patch of haemocytes: mantle |
no effect
small patch of haemocytes: mantle small patches of haemocytes: mantle & siphon |
| March 9 | 5 | 4
5 6 |
small patches of haemocytes: mantle
small patches of haemocytes: mantle & siphon small patches of haemocytes: mantle |
large pustules: mantle & siphon
wart near external surface: mantle large pustules: mantle & siphon |
| March 11 | 7 | 7
8 9 |
small wart being ejected: mantle
small patches of haemocytes: mantle patches of haemocytes throughout b |
large pustules: mantle & siphon b
large wart being ejected: mantle & siphon b large wart being ejected: mantle & siphon |
| March 13 | 9 | 10
11 12 |
small wart being ejected: siphon
small patches of haemocytes: mantle & siphon patches of haemocytes throughout b |
large wart being ejected: mantle & siphon
large wart being ejected: mantle & siphon healed wart on surface: siphon |
| March 20 | 16 | 13
14 15 |
patches of haemocytes throughout b
no effect no effect |
large wart being ejected: mantle & siphon
large wart being ejected: mantle & siphon large wart being ejected: mantle, siphon |
| March 27 | 23 | 16
17 18 |
large wart being ejected: mantle & siphon b
wart out, healed epithelium: siphon patch of haemocytes under healed wart: siphon |
|
| April 3 | 30 | 19
20 21 |
large pustules: mantle & siphonb
wart out, healed epithelium: siphon wart out, healed epithelium: mantle & siphon |
b Bacteria were observed in association with haemocyte concentrations.
The bacteria that were observed intermittently in both groups starting on day seven post inoculation (Table 4) are thought to be a contaminant, probably mechanically introduced during the injection process. This experiment demonstrates that, regardless of the provocation, the geoduck clam reacts in a similar manner. The presence of a foreign protein, bacteria, or material collected from a ‘wart’ produced an aggregate of haemocytes and necrosis in the musculature which are eventually ejected through the epithelium to the exterior. Figures 9 and 10 are of histological preparations at day nine post inoculation of clam number 10 from Groups B and D respectively. In the two figures, the similarity of reaction to very different inoculated material is evident. In both cases there is an aggregation of haemocytes remaining in the musculature of the siphon and a ‘wart’ formed of dead and dying granular haemocytes partly surrounded by the periostracum. An etiological agent was not detected in any of the histological preparations.
Figures 9 and 10. Histological sections through ‘warts’ on the surface of the siphon of a juvenile geoduck clams induced experimentally on day 9 post inoculation. Haematoxylin and eosin stain.

Figure 9. 'Wart' (w) and concentration of haemocytes still within the musculature (arrow) resulting from the inoculation with foetal calf serum.

Figure 10. 'Wart' (w) and concentration of haemocytes still within the musculature (arrow) resulting from inoculation with ‘wart’ material from a naturally infected adult geoduck clam.
Conclusion
Most inoculated geoduck clams developed pustules reminiscent of ‘warts’ found on the wild adults. The development of these pustules on the controls indicates that ‘warts’ are a consequence of the response of the geoduck clam to foreign material. It remains unresolved if the ‘warts’ in wild geoduck clams are the result of the response to an invading infectious pathogen or to mechanical damage that caused some debris to be accidentally lodged within the tissue. To rule out the possibility that infectious agent(s) cause the problem and to determine if the cause is the same throughout British Columbia, further research is required.
References
Fisheries Management Area locations as mentioned in above text can be viewed on the map.
Morse, M.P. and Zardus, J.D. 1997. Bivalva. Microscopic Anatomy of Invertebrates Vol. 6A Mollusca II. F.W. Harrison and A.J. Kohn. Wiley-Liss. pp. 7-118.
Simkiss, K. 1988. Molluscan Skin (excluding Cephalopods). The Mollusca Vol. 11 Form and Function. E.T. Truman and M.R. Clarke. Academic Press Inc. pp. 11 - 35.
Citation Information
Bower, S.M. and Blackbourn, J. (2003): Geoduck clam (Panopea generosa): Anatomy, Histology, Development, Pathology, Parasites and Symbionts: 'Wart' Transmission Experiments.
Date last revised: August 2020
Comments to: Susan Bower
- Date modified: