Labyrinthulochytrium haliotidis of Abalone
On this page
Category
Category 2 (In Canada and of Regional Concern)
Common, generally accepted names of the organism or disease agent
Infection of the head and foot by Labyrinthulochytrium (=Apanochytrium, =Labyrinthuloides) haliotidis.
Scientific name or taxonomic affiliation
Originally placed in the genus Labyrinthuloides, L. haliotidis was transferred to the genus Aplanochytrium when the two genera were synonymised (Leander and Porter 2000). Hassett and Gardinger (2018) addressed the problem of polyphyly within the aplanochytrids by erecting a new genus Labyrinthulochytrium and included the former A. haliotidis within that genus. According to GenBank (as of March 2020), the full lineage of this parasite is: Eukaryota; Stramenopiles; Labyrinthulomycetes; Thraustochytriaceae; Labyrinthulochytrium (Taxonomy ID: NCBI:txid38760).
Geographic distribution
West coast of Canada abalone mariculture facility.
Host species
Juvenile Haliotis kamtschatkana and Haliotis rufescens.
Impact on the host
Destruction of head and foot tissues and subsequent mortality of up to 100% of the juvenile abalone (less than 4 mm in shell length and younger than 6 months old) being cultured in raceways. This parasite contributed to the demise of the only attempt at commercially culturing abalone in British Columbia, Canada during the 1980's. Abalone greater than 5 mm in shell length demonstrated increased resistance to infection and mortality. Abalone about 25 mm in shell length could not be infected even when L. haliotidis was inoculated into the foot muscle. Intact small Pacific oysters (Crassostrea gigas) and scallops (Patinopecten yessoensis) (between 5 and 10 mm in shell length) were also resistant to infection. However, a few oysters with badly cracked shells became infected, indicating that L. haliotidis is capable of using living oyster tissue as a source of nutrients for growth and multiplication, if it can gain access to tissue not protected by cilia. Labyrinthulochytrium haliotidis grew well on a wide variety of aseptic nutrient medium and could also use pine pollen as a source of nutrients for vegetative multiplication.
Diagnostic techniques
Gross Observations
Swelling of abalone foot and head.
Wet Mounts
Numerous spherical protozoa (about 10 µm in diameter) throughout tissues of head and foot. However, due to the morphological similarities of L. haliotidis to other thraustochytrids, such as Labyrinthulochytrium arktikum isolated from seawater samples collected from the nearshore marine environment outside of Tromsø, Norway that were baited with Pinus sp. (pine) pollen (Hassett and Gardinger 2018), identification of the parasite outside its host is currently limited to molecular analysis.
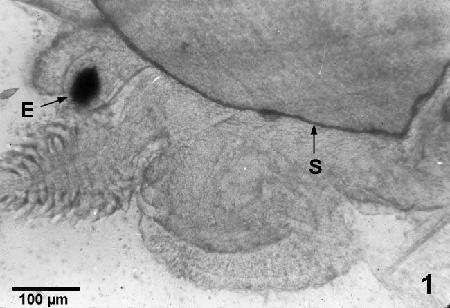
Figure 1. Wet mounted, unstained preparation of the head region of a small uninfected juvenile abalone (Haliotis kamtschatkana) showing the eye (E) and shell (S).

Figure 2. Wet mounted, unstained preparation of a small infected juvenile abalone (Haliotis kamtschatkana) showing numerous Labyrinthulochytrium haliotidis (P) liberated from and embedded in tissues of the head region; also shown are the swollen eye (E) and crushed pieces of shell (S) of the abalone.
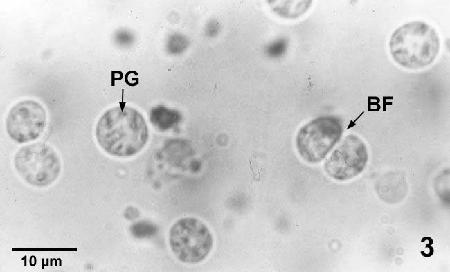
Figure 3. Labyrinthulochytrium haliotidis liberated from an infected abalone showing the dark, light-refractive granules (PG) and one specimen undergoing binary fission (BF).
Histology
Presence of numerous L. haliotidis in cross-section of the head and foot of small abalone.

Figure 4. Haematoxylin and eosin stained histological section of a juvenile abalone (Haliotis kamtschatkana) with Labyrinthulochytrium haliotidis (arrows) within the nerve ganglion and in surrounding tissues.
Immunological Assays
Fluorescent antibodies were produced and showed promise in facilitating the detection of this parasite. However, this technique has not been fully tested to verify its specificity.
Culture
Parasites were cultured in minimum essential medium (MEM) with the production of biflagellate zoospores when the cultured vegetative stage was transferred to sterile sea water.
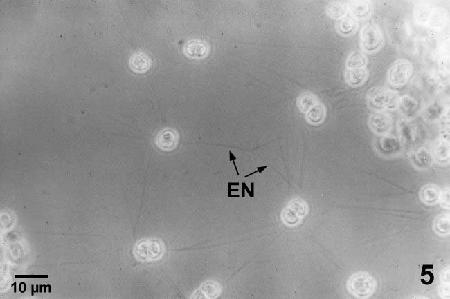
Figure 5. Living unstained culture of Labyrinthulochytrium haliotidis growing on a glass slide in a culture of minimum essential medium with 10% fetal calf serum. Note the ectoplasmic nets (EN) radiating from the individuals of the colony.
Electron Microscopy
Labyrinthulochytrium haliotidis has organelles that are unique to the thraustochytrids. The vegetative stage, both within the abalone host and in aseptic cultures has sagenogenetosomes on the cell surface from which the ectoplasmic membrane net originate and an outer membranous wall from which scales slough. The ectoplasmic membrane net was used to obtain nutrients distant from the parasite and for locomotion. When the vegetative stage of L. haliotidis was transferred to sea water, it transformed into a zoosporoblast within which about 12 zoospores developed. Within the zoosporoblast, each developed zoospore was enclosed in a membrane and the zoosporoblast wall was composed of scales. The zoospores became active and ruptured the zoosporoblast within 24-72 hours of vegetative cells being transferred from nutrient media to sea water at 10° C. Each zoospore had scales that sloughed from the outer membranous wall and two laterally attached flagella. The anterior flagellum was adorned with a brush of fine fibers called mastigonemes and the shorter posterior flagellum was glabrous and had a tapered tip. The zoospore actively swam for about 24 hours then sloughed the flagella. The resulting cell remained viable for at least two years in sterile sea water at 4° C.

Figure 6. Transmission electron micrograph of a Labyrinthulochytrium haliotidis within the muscle tissue of a juvenile abalone (Haliotis kamtschatkana) showing the ectoplasmic net (EN) originating from the sagenogenetosome, scales (SC) sloughing from the outer membrane wall, nucleus (N), mitochondria (M) and golgi apparatus (GD). Uranyl acetate and lead citrate stain.
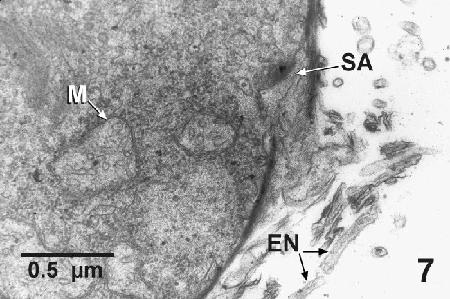
Figure 7. Transmission electron micrograph of a Labyrinthulochytrium haliotidis from liquid culture media showing the sagenogenetosome (SA) on the periphery of a cell that was actively producing an ectoplasm net (EN), which consist of a unit membrane with no evidence of cell organelles internally. Mitochondrial profiles (M) containing tubular cristae are evident adjacent to the sagenogenetosome. Uranyl acetate and lead citrate stain.
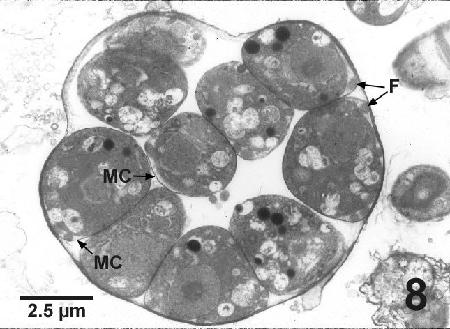
Figure 8. Transmission electron micrograph of a cultured Labyrinthulochytrium haliotidis zoosporoblast from sea water containing well-developed zoospores, indicated by the presence of flagella (F). Each zoospore is closely surrounded by its own membrane covering (MC). Uranyl acetate and lead citrate stain.

Figure 9. Transmission electron micrograph of the edge of a zoosporoblast of Labyrinthulochytrium haliotidis illustrating the scale nature of the outer wall (SC) and mastigonemes (M) extending from about one third of the cross sectional surface of the anterior flagellum (F) of a developed zoospore within the zoosporoblast. Uranyl acetate and lead citrate stain.
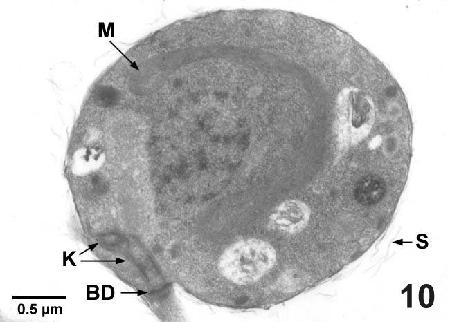
Figure 10. Transmission electron micrograph of a zoospore of Labyrinthulochytrium haliotidis from sea water illustrating the kinetosome (K), the complex terminal plate - basal disc (BD), the horse-shoe shaped mitochondrion (M) around the nucleus and scales (SC) sloughing from the outer membranous wall. Uranyl acetate and lead citrate stain.

Figure 11. Scanning electron micrograph of a zoospore of Labyrinthulochytrium haliotidis from sea water. Note the subapical attachment site of the two flagella, the coarse texture of the longer anterior flagellum where debris has attached to the mastigonemes and the thin tapered tip of the short posterior flagellum.
Molecular Characteristics
Leipe et al. (1996) submitted a 1846 base pair sequence of the 18S ribosomal RNA which they referred to as 16S-like rDNA of Labyrinthulochytrium haliotidis to GenBank (Accession U21338, version U21338.1). Subsequently. Leander and Porter (2001) reported that the sequence of L. haliotidis submitted to GenBank “was sequenced from nonviable material (L. Goggin pers com) and may need to be reisolated and resequenced to verify any phylogenetic position. It is likely that the sequence may actually be that of a thraustochytrid contaminant. Since this organism is a parasite of abalone in mariculture and there are currently no known outbreaks of disease, L. haliotidis is currently unavailable for further study” (Leander and Porter 2001). However, Hassett and Gardinger (2018) described another thraustochtyrid with a 18S small ribosomal subunit coding region that had closest molecular affinity to L. haliotidis with the GenBank accession number U21338.1 submitted by Leipe et al. (1996). To resolve the problem of polyphyly encountered by Leander and Porter (2001) and other investigators, Hassett and Gardinger (2018) created a new genus (Labyrinthulochytrium) for their new species and L. haliotidis. A partial sequence (216 base pairs) of the 18S (small subunit) ribosomal RNA gene of L. haliotidis is also available in GenBank, (Accession J842400.1).
Methods of control
Abalone from areas with records of the disease should not be imported into areas with no record of L. haliotidis. This parasite is resistant to many disinfectants but it can be destroyed by a 20 minute exposure to 25 mg chlorine per litre of sea water. Treating sea water with at least 0.97 mg ozone per litre for 25 min can destroy many (but not all) of the zoospores. However, the proper authorities should be consulted regarding prevention or control methods.
References
- Bower, S.M. 1987a. Labyrinthuloides haliotidis n. sp. (Protozoa: Labyrinthomorpha), a pathogenic parasite of small juvenile abalone in a British Columbia mariculture facility. Canadian Journal of Zoology 65: 1996-2007.
- Bower, S.M. 1987b. Pathogenicity and host specificity of Labyrinthuloides haliotidis (Protozoa: Labyrinthomorpha), a parasite of juvenile abalone. Canadian Journal of Zoology 65: 2008-2012.
- Bower, S.M. 1987c. Artificial culture of Labyrinthuloides haliotidis (Protozoa: Labyrinthomorpha), a pathogenic parasite of abalone. Canadian Journal of Zoology 65: 2013-2020.
- Bower, S.M. 1989. Disinfectants and therapeutic agents for controlling Labyrinthuloides haliotidis (Protozoa: Labyrinthomorpha), an abalone pathogen. Aquaculture 78: 207-215.
- Bower, S.M. 2000. Infectious diseases of abalone (Haliotis spp.) and risks associated with transplantation. In: Campbell, A. (Editor), Workshop on Rebuilding Abalone Stocks in British Columbia. Canadian Special Publication of Fisheries and Aquatic Sciences 130: 111-122.
- Bower, S.M. 2003. Update on emerging abalone diseases and techniques for health assessment. Journal of Shellfish Research 22: 805-810.
- Bower, S.M. 2005. Labyrinthomorpha (labyrinthomorphs). In: Rohde, K. (ed.), Marine Parasitology. CSIRO Publishing, Collingwood, pp. 20-23.
- Bower, S.M., D.J. Whitaker and R.A. Elston. 1989. Detection of the abalone parasite Labyrinthuloides haliotidis by a direct fluorescent antibody technique. Journal of Invertebrate Pathology 53: 281-283.
- Bower, S.M., D.J. Whitaker and D. Voltolina. 1989. Resistance to ozone of zoospores of the thraustochytrid abalone parasite, Labyrinthuloides haliotidis (Protozoa: Labyrinthomorpha). Aquaculture 78: 147-152.
- Bower, S.M., N. McLean and D.J. Whitaker. 1989. Mechanism of infection by Labyrinthuloides haliotidis (Protozoa: Labyrinthomorpha), a parasite of abalone (Haliotis kamtschatkana) (Mollusca: Gastropoda). Journal of Invertebrate Pathology 53: 401-409.
- Hassett, B.T. and R. Gradinger. 2018. New species of saprobic Labyrinthulea (=Labyrinthulomycota) and the erection of a gen. nov. to resolve molecular polyphyly within the Aplanochytrids. Journal of Eukaryotic Microbiology 65: 475-483.
- Leander, C.A. and D. Porter. 2000. Redefining the genus Aplanochytrium (Phylum Labyrinthulomycota). Mycotaxon 76: 439-444 (see details on pg. 442).
- Leander, C.A. and D. Porter. 2001. The Labyrinthulomycota is comprised of three distinct lineages. Mycologia 93: 459-464.
- Leipe, D.D., S.M. Tong, C.L. Goggin, S.B. Slemenda, N.J. Pieniazek and M.L. Sogin. 1996. 16S-like rDNA sequences from Developayella elegans, Labyrinthyloides haliotidis and Proteromonas lacertae confirm that the stramenopiles are a primarily heterotrophic group. European Journal of Protistology 32: 449-458.
Citation information
Bower, S.M. (2019): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Labyrinthulochytrium haliotidis of Abalone.
Date last revised: March 2019
Comments to Susan Bower
- Date modified: