Protistan Pathogen of Pandalid Shrimp (SPP)
On this page
Category
Category 2 (In Canada and of Regional Concern)
Common, generally accepted names of the organism or disease agent
Spot prawn parasite (SPP) originally thought to be a Hematodinium-like organism of prawns. Note: Hematodinium spp. have been described from various marine crustaceans including crabs from the Atlantic Ocean, Chionecetes spp. from the north Pacific and north Atlantic oceans, crabs from the vicinity of Australia, and lobsters.
Scientific name or taxonomic affiliation
A eukaryotic parasite of uncertain affiliation with superficial similarity to parasitic dinoflagellates (large plasmodia and numerous trophonts) but with a different mechanism of nuclear division and lack of organelles characteristic of parasitic dinoflagellates. Also, the antigenic characteristics were not consistent with those of other parasitic dinoflagellates (Dungan et al. 2000; Bushek et al. 2002). Molecular analysis suggests association with Haplosporidia (Reece et al. 2000, 2004). However, morphological features characteristic of the Haplosporidia were not observed in SPP.
Geographic distribution
Strait of Georgia in the vicinity of Powell River, British Columbia, Canada (Bower and Meyer 2002), and Yakutat and Prince William Sound in Alaska, USA (Meyers et al. 1994).
Host species
Pandalus platyceros and Pandalus borealis.
Impact on the host
This disease was first reported in British Columbia by commercial prawn harvesters who found prevalences of clinical infection up to 10% in September 1991. In an area with clinical infections of 0.6%, subclinical infections were detected in 19% of 100 spot prawns examined histologically in 1992. In same season and area in 1993, clinical infections had increased to 2.2% but subclinical infections were 14.1% for a calculated prevalence of 16.3% (reduced from prevalance detected in 1992). In subsequent years, the prevalence of infection continued to decline to only 2.5% in 1995 and remained low when last sampled in 1998. In most P. platyceros with subclinical infections, the duration of the infection had been sufficiently long to affect gonadal development and the morphology of secondary sexual characteristics (the appendix masculina and appendix interna on the endopod of the second pleopods were abnormal). Infected prawns from the field did not survive in captivity. In Alaska, unconfirmed reports were of prevalences reaching 50%. However, documented prevalences of clinical infections were commonly less than 1% (Meyers et al. 1994).
Diagnostic techniques
Gross Observations
Prawns with clinical infections are lethargic, have an unusual orange-pink colour in life and, a characteristic milky-like fluid pours from the cephalothorax when the prawn is decapitated.
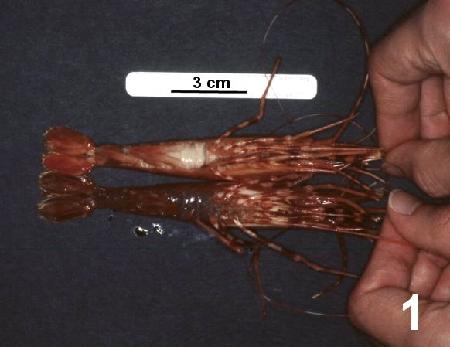
Figure 1. Pandalus platyceros with unusual orange colouration and with "milky" haemolymph evident through the thin cuticle on the ventral surface of the abdomen (see spot prawn next to scale bar) caused by the spot prawn parasite (SPP) in comparison to a normal (uninfected) spot prawn (lower specimen).
Wet Mounts
Haemolymph is full of either round or discoid shaped (reminescent of mammalian red blood cells but larger in size) protozoa about 7 µm in diameter in prawns with clinical infections.
Histology
Numerous protozoa filling all haemal sinuses. In prawns with subclinical infections, large multinucleate plasmodia were observed in the connective tissue adjacent to the digestive gland.
Figures 2 to 7 illustrate developmental stages of the SPP in histological sections of Pandalus platyceros stained with haematoxylin and eosin stain.
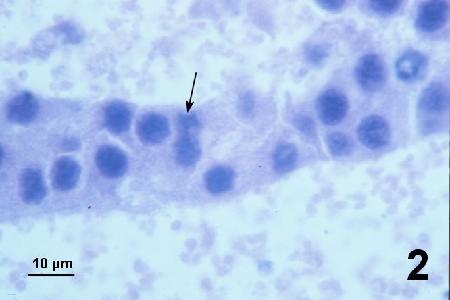
Figure 2. Immature plasmodia with poorly defined basophilic nuclei. Nuclear division indistinct with nuclei elongated to form a basophilic smudge prior to fission (arrow).
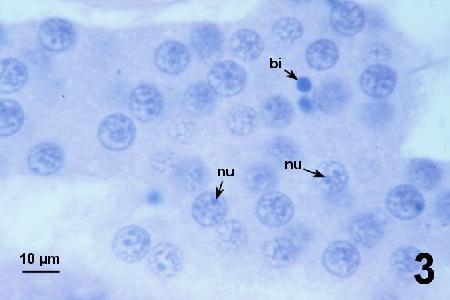
Figure 3. Mature plasmodium filling the tissue sinus and containing abundant nuclei showing a peripheral chromatin ring, an internal chromatin web and up to three (usually two) tiny nucleoli (nu). Dense basophilic inclusions (bi) of unknow significance were observed in some specimens from some spot prawns.
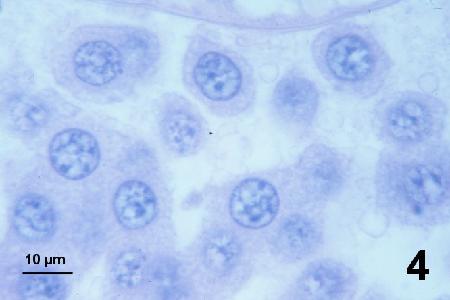
Figure 4. Mature plasmodium in the process of fragmentation into trophonts of various shapes.
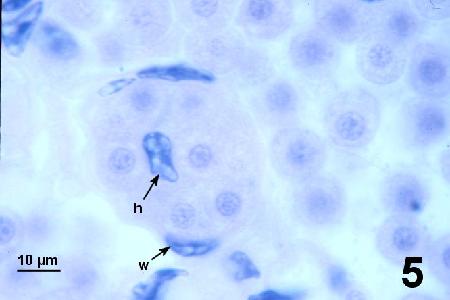
Figure 5. Spherical trophonts with a central round nucleus similar in morphology but slightly smaller in diameter than nuclei in mature plasmodia. Overwhelming numbers of SPP occur outside and within the vascular system (w indicates a nucleus of the haemal vessel wall). Haemocytes (h indicates the nucleus of a haemocyte in the vascular sinus) were rarely observed.
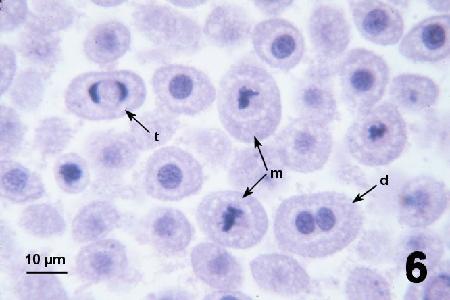
Figure 6. Trophonts in the process of binary fission showing metaphase (m), late telophase (t) and one cells in which the nucleus has recently divided (d).
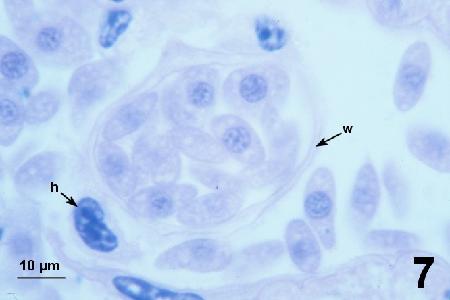
Figure 7. Discoid trophonts with a central round nucleus similar to that of spherical trophonts.Haemocytes (h indicates the nucleus of a haemocyte in the tissue sinus) were rarely observed, and SPP occurred outside and within the vascular system (w indicates the wall of the haemal vessel).
The developmental cycle was proposed from the histological examination of 270 infected spot prawns. The immature plasmodia (Fig. 2) grow into large plasmodia that fill most tissue sinuses including spaces between the digestive gland tubules. At a subsequent stage, some nuclei acquire characteristics of mature plasmodia (Fig. 3) and no longer divide. However, depending on the stage of development, plasmodia (or regions of a plasmodium) in some areas of the spot prawn (e.g., at the centre of the digestive gland or base of the pereiopod coxae) remain immature with dividing nuclei for some time. In other areas of the spot prawn (e.g., in the periphery of the digestive gland, heart and gills) the large plasmodia may start segmenting into trophonts (Fig. 4). Apart from abnormal morphology of the endopod on the second pleopod, spot prawns at the plasmodial stage of infection did not show visible signs of disease, unless the plasmodia were very large and in the process of segmenting into trophonts. All spot prawns with visible disease (unusual orange coloration and white haemolymph) had overwhelming heavy infections made up of a plethora of trophonts that gave the haemolymph a milky appearance.
The trophonts produced by the segmentation of the large mature plasmodia initially appeared variable in shape (Fig. 4) but eventually formed spheres. In 10 (4%) of the infected spot prawns, all SPP observed were spherical trophonts (Fig. 5) and plasmodia were no longer present. Sixteen (6%) of the infected spot prawns had spherical trophonts in the process of binary fission (Fig.6) and in three of these spot prawns, over 30% of the SPP were undergoing binary fission. Forty-two (16%) of the infected spot prawns had varying percentages of discoid (Fig. 7) and spherical (Fig. 5) trophonts. Because discoid trophonts never occurred in prawns with plasmodia, these cells were considered the most "mature" form of SPP that was observed.
Electron Microscopy
Ultrastructural features believed to be either unique to SPP or representative of related species include the process of binary fission in the trophont stage (Fig. 6), nucleus-associated organelles and surface extensions in some specimens as illustrated below.
Figures 8 to 14. Electron micrographs of SPP fixed as indicated in each figure caption and stained with uranyl acetate and lead citrate stain.
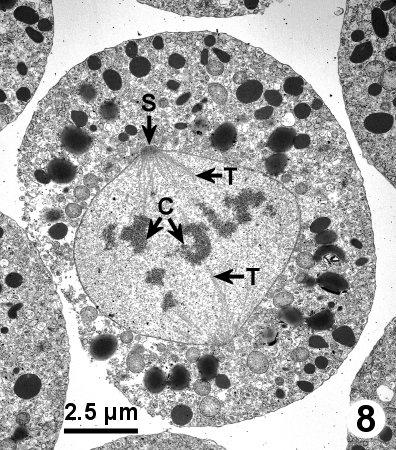
Figure 8. A spherical trophont in late metaphase or early anaphase with an intact nuclear membrane surrounding condensed chromosomes (C) that are connected by microtubutles (T) to spindle pole bodies (S) emerging through the nuclear envelope. Tissue fixed in 4% glutaraldehyde in Millonig's phosphate buffer.

Figure 9. Magnification of Fig. 8 illustrating the microtubules connecting to the spindle pole body at a gap in the nuclear membrane.
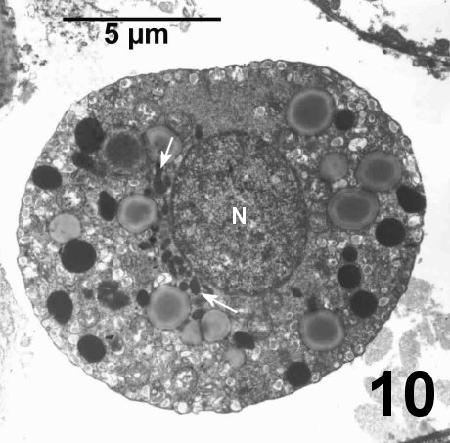
Figure 10. Cluster of nucleus-associated organelles (between arrows) on the outer periphery of the nucleus. Tissue fixed in 4% glutaraldehyde in Millonig's phosphate buffer.

Figure 11. Nucleus-associated organelles, clustered in a depression in the nucleus (N), appear to contain a few tubules in a fine osmophilic matrix when preserved with 2.5% glutaraldehyde in sea water.

Figure 12. Replicate sample from the same infected prawn as illustrated in Fig. 11 that was preserved in 2.5% glutaraldehyde in Cacodylate buffer showed a coarse matrix and appeared to contain numerous tubules and membranes. The nucleus (N) of this SPP has several membrane pores (P) and an unusual extension (E) of the nuclear membrane. The morphology of the nucleus-associated organelles can be compared to that of a mitochondrium (M) in the SPP cytoplasm.

Figure 13. Numerous ectoplasmic membrane tubules between the outer membrane of the parasite (m indicates a SPP mitochondrium) and the host tissue. Tissue fixed in 2.5% glutaraldehyde in filtered sea water.
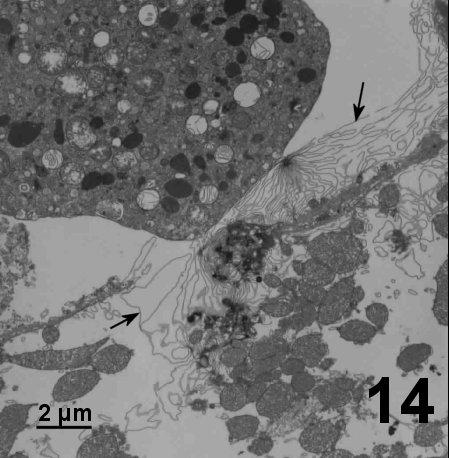
Figure 14. Tubules compressed into multiple membrane sheets (arrows) that extend between the numerous mitochondria of a lysed myocyte in the heart of a spot prawn. Tissue fixed in 2.5% glutaraldehyde in filtered sea water.
Methods of control
No known methods of treatment or control. Attempts to transmit SPP between spot prawns in the laboratory by inoculation and cohabitation were unsuccessful suggesting that other host(s) may be involved and/or other developmental stages occur in the life cycle. Alternately, the spot prawns may be an abnormal host in the life cycle of this parasite. The apparent disintegration of the nucleus in trophonts of SPP in some spot prawns with overwhelming infections and the rapid loss of parasites in dead prawns used as a source of infection during cohabitation experiments may suggest that SPP is not well adapted to the spot prawn.
References
Bower, S.M. and G.R. Meyer. 1996. A Hematodinium-like dinoflagellate parasite of prawns, Pandalus platyceros, in British Columbia. Bulletin of the Canadian Society of Zoologists 27: 43-44.
Bower, S.M. and G.R. Meyer. 2000. Description of an unusual parasite in prawns, Pandalus platyceros, in British Columbia, Canada. Journal of Shellfish Research 19: 642. (Abstract).
Bower, S.M. and G.R. Meyer. 2002. Morphology and ultrastructure of a protistan pathogen in the haemolymph of shrimp (Pandalus spp.) in the northeastern Pacific Ocean. Canadian Journal of Zoology 80: 1055-1068.
Bower, S.M., G.R. Meyer and J.A. Boutillier. 1993. Diseases of spot prawns Pandalus platyceros caused by an intracellular bacterium and a Hematodinium-like protozoa. Journal of Shellfish Research 12: 135.
Bushek, D., C.F. Dungan and A.J. Lewitus. 2002. Serological affinities of the oyster pathogen Perkinsus marinus (Apicomplexa) with some dinoflagellates (Dinophyceae). The Journal of Eukaryotic Microbiology 49: 11-16.
Dungan, C.F., R. Hamilton, D. Bushek, J. Cardinal and A. Lewitus. 2000. Serological affinities between Perkinsus marinus and some parasitic dinoflagellates. Journal of Shellfish Research 19: 643-644. (Abstract).
Meyers, T.R., D.V. Lightner and R.M. Redman. 1994. A dinoflagellate-like parasite in Alaskan spot shrimp Pandalus platyceros and pink shrimp P. borealis. Diseases of Aquatic Organisms 18: 71-76.
Reese, K.S., E.M. Burreson, S.M. Bower and C.F. Dungan. 2000. Molecular analyses of a parasite in prawns (Pandalus platyceros) from British Columbia, Canada. Journal of Shellfish Research 19: 647. (Abstract).
Reece, K.S., M.E. Siddall, N.A. Stokes and E.M. Burreson. 2004. Molecular phylogeny of the haplosporidia based on two independent gene sequences. The Journal of Parasitology 90: 1111-1122.
Citation Information
Bower, S.M. (2002): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Protistan Pathogen of Pandalid Shrimp (SPP).
Date last revised: November 2002
Comments to Susan Bower
- Date modified: