Phototrophic Endolith Invasion of Mussel Shells
On this page
Category
Category 1 (Not Reported in Canada)
Common, generally accepted names of the organism or disease agent
Mussel microbial endoliths, Blue-green alga shell infestation.
Scientific name or taxonomic affiliation
Various species of filamentous cyanobacteria including Plectonema terebrans, Hyella caespitosa, Mastigocoleus testarum, Mastigocoleus sp. (Nostochopsidaceae), and aggregated (pseudofilamentous) cyanobacterium Pleurocapsa sp.
Geographic distribution
Coast of South Africa, especially along the Western Cape from Saldanha Bay to Simonstown and wave-exposed coastline in the Bathurst and Peddie districts. Similar infestations reported from Sierra Leone.
Host species
Mytilus galloprovincialis introduced about 1970 for culture purposes (currently well established in the wild), and Perna perna as well as other native species of mussels (Choromytilus meridionalis, Aulacomya ater).
Impact on the host
Shell strength is compromised by the numerous tiny burrows created by the endolithic cyanobacteria. Mussels with weakened shells are more vulnerable to predation and mechanical effects of wave action. Heavy infestation may result in fracture holes forming in the shell and is soon followed by mussel death. Prevalence varied from 94% at high intertidal sites to 1% at subtidal sites and prevalence and intensity increased with shell length. A correlation was observed between heavy infestation and environmental factors of high population density, high tidal position, and high wave action (Webb and Korrûbel 1994). The loss of the periostracum, (varnish-like protein layer that covers the shell) which provides protection to the mineral shell underneath, seems to predispose mussels to infestation (Kaehler 1999). Infestation and resulting damage was most severe in intertidal M. galloprovincialis. However, M. galloprovincialis from a hanging culture facility in an epizootic area were virtually free of the infestation. The severity of infestation and damage was less in native species of mussels except for the ribbed mussel (A. ater) at Port Nolloth where severe shell weakness was common (Webb and Korrûbel 1994). The report from Sierra Leone and all previous reports unanimously indicated that direct damage resulting from infestation of bivalve shells is usually negligible. However, the interaction of a combination of factors (i.e., erosion of the periostracum, successional sequence of colonists and mechanical properties of the shell) may result in phototrophic endoliths causing severe shell degradation and eventually mussel mortality (Kaehler 1999). Also, the presence of endoliths resulted in a marked increase in shell regeneration (which usually could not compensate for rapid endolith-induced shell degradation especially around the site of adductor muscle attachment) and a reduction in the reproductive output of infested individuals (Kaehler and McQuaid 1999).
Diagnostic techniques
Gross Observations
Focal loss of periostracum in conjunction with gray corroded patches on the shell surface and loss of distinct outer growth lines (i.e., shells have distinct discolouration and shell surface erosion). In extreme cases, shells are extremely pitted, deformed and brittle, and large fracture holes, most frequently occurring over the adductor muscle attachment area, are observed.
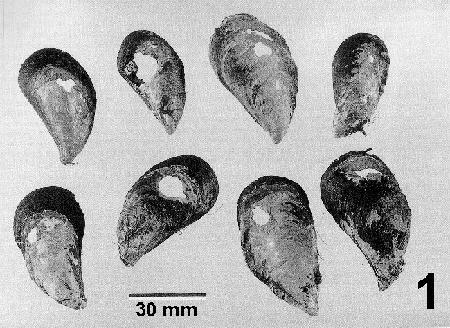
Figure 1. Mytilus galloprovincialis showing shell damage and fracture holes caused by Mastigocoleus sp. Image from Webb and Korrûbel 1994. J. Shellfish Res. 13: 12.
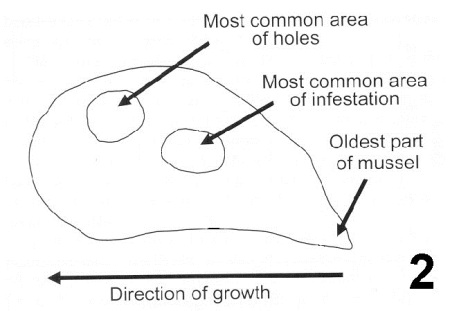
Figure 2. Summary drawing indicating the most common area of Mastigocoleus sp. infestation on the shell of Mytilus galloprovincialis and most common area where holes occur. Image from Webb and Korrûbel 1994. J. Shellfish Res. 13: 16.
Wet mount
Shell fragments decalcified by immersion in 10% acetic acid overnight are teased apart to reveal the segmented chains of endolithic cyanobacteria.
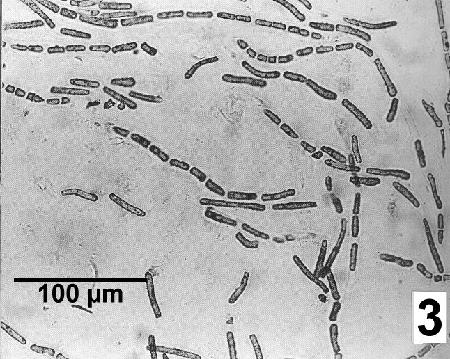
Figure 3. Mastigocoleus sp. filaments after the release from the shell of Mytilus galloprovincialis by decalcification. Image from Webb and Korrûbel 1994. J. Shellfish Res. 13: 13.
Histology
To identify cell morphology of the endolithic cyanobacterium, section shells (5 mm thick) with a diamond saw, followed by fixation in 4% formalin, partially decalcification in 5% acetic acid and thin sectioning with a freezing microtome.After full decalcification, mount on a microscope slide (for details see Kaehler 1999) and examine by phase contrast microscopy.
Scanning Electron Microscopy
The filamentous thallus of endolithic cyanobacteria can be observed in burrows about 8 µm in diameter giving the shell matrix an honeycomb appearance.
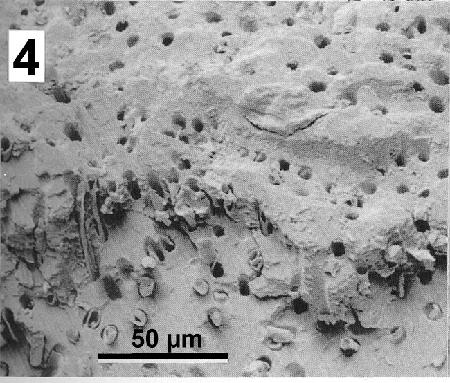
Figure 4. Scanning electron micrograph of the shell of Mytilus galloprovincialis infested with Mastigocoleus sp. Image from Webb and Korrûbel 1994. J. Shellfish Res. 13: 13.
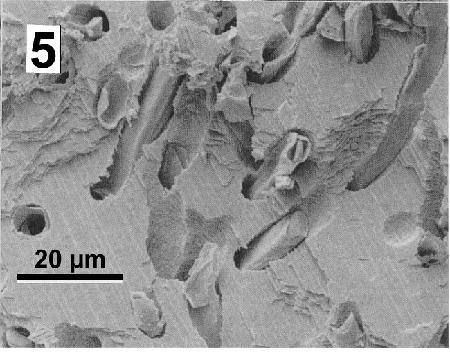
Figure 5. Details of Mastigocoleus sp. tunnels in a mussel shell. Image from Webb and Korrûbel 1994. J. Shellfish Res. 13: 13.
The morphology of the shell erosion can be visualised in tunnel-cast sections. Clean shells sections, (5 mm thick prepared with a diamond saw), in sodium hypochlorite solution, and embed in araldite resin at 40°C under vacuum. After the resin has polymerised, re-section, dissolve the carbonate in acetic acid, and gold sputter-coat the resulting resin casts (for details see Kaehler 1999).
Methods of control
No known method of prevention. However, because: 1) fast growing subtidal mussels were least infested, 2) phototrophic endolith growth seems to be limited by reduced exposure to light, 3) algal distribution is limited by desiccation and 4) endolith colonisation tends to be related to the exposure of calcarious shell through the removal of protective periostracum, management techniques may be implemented to reduce the severity of the disease if it occurs in cultured mussels.
References
Kaehler, S. 1999. Incidence and distribution of phototrophic shell-degrading endoliths of the brown mussel Perna perna. Marine Biology 135: 505-514.
Kaehler, S. and C.D. McQuaid. 1999. Lethal and sub-lethal effects of phototrophic endoliths attacking the shell of the intertidal mussel Perna perna. Marine Biology 135: 497-503.
Webb, S.C. and J.L. Korrûbel. 1991. Blue-green alga attacks mussels: threat to rock lobster resource? South African Shipping News & Fishing Industry Review 46: 30-31.
Webb, S.C. and J.L. Korrûbel. 1994. Shell weakening in marine mytilids attributable to blue-green alga Mastigocoleus sp. (Nostochopsidaceae). Journal of Shellfish Research 13: 11-17.
Citation Information
Bower, S.M., Korrûbel, J.L., Webb, S.C. (2002): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Phototrophic Endolith Invasion of Mussel Shells
Date last revised: September 2002
Comments to Susan Bower
- Date modified: