Aquaculture Collaborative Research and Development Program (ACRDP) Fact Sheet
Issue 4 -
April, 2010
Summary
Oysters should be grown in waters containing minimal numbers of microbes which may be potentially pathogenic to the oysters or, from a food safety point of view, to the humans that consume them. Influxes of birds or marine mammals that occupy rafts or floats at a shellfish farm, may lead to increased fecal material in the water and an accumulation of fecal coliforms and other human pathogens in the shellfish. Contaminations that result in farm closures or harm to humans can cause serious damage to the business and reputation of the shellfish industry. Therefore, a cost-effective method of decreasing fecal source bacterial and other microbes in seawater would be extremely beneficial for shellfish and other aquaculture-based industries. This project examined whether microbubblers can reduce microbial contaminants in seawater.
Experiments were conducted to evaluate the survival of various bacteria in seawater in the presence and absence of different microbubblers and aeration devices. Contrary to the indications of previous observations, microbubblers did not directly affect the survivability of selected potential microbial contaminants of oysters. Decreases in bacterial counts were found in tested waters when a microbubbler aeration device containing some metallic parts was used. An accumulation of copper leaching from the metal parts in the device, as opposed to the production of micro-bubbles by the device, could explain the rapid decline of bacteria seen in laboratory tests.
Introduction
The microbial flora of oysters, like that of other filter feeders, is largely determined by the microbial flora of the water that they inhabit. It is important for oysters to be surrounded by water containing minimal numbers of microbes that may be potentially pathogenic to the oysters or, from a food safety point of view, to the humans who consume them. However, for almost all shellfish farms, birds alighting upon structures and seals or sea lions occupying floats and rafts are common occurrences. An influx of these animals at the shellfish farm may lead to an accumulation, in the shellfish, of fecal coliforms ( e.g., Escherichia coli) and human pathogens such as Vibrio parahaemolyticus (gastroenteritis), Salmonella spp., and other foodborne illnesses.
In Canada, the levels of coliform bacteria permitted in shellfish culture water and in the shellfish itself are regulated by Environment Canada and the Canadian Food Inspection Agency. If significant bacterial contamination of shellfish or the culture waters is detected, closure of the oyster farm may occur. Of even greater concern is the possibility that contaminated oysters may not be detected by random inspections and may adversely affect the health of the final consumer. In either case, serious damage to the business and reputation of the shellfish industry would occur. A cost-effective method of accelerating the decrease in fecal source bacterial numbers in seawater would be extremely useful for shellfish and other aquaculture-based industries.
Water aeration is an important aspect of aquaculture operations. Devices that generate minute or microscopic bubbles have been used in fish farming operations to provide adequate dissolved oxygen for production under anoxic conditions. Micro-bubbles are ultra-fine gas bubbles of less than 50 µm diameter which are produced when air is sparged through, or introduced into, water. A variety of devices referred to as "microbubblers", which produce such micro-bubbles, are commercially available.
Early trials indicated that one type of microbubbler device caused a rapid reduction in fecal coliform numbers in both seawater and in oyster tissue. This study examined the potential of microbubblers to rapidly reduce the numbers of cultivable fecal coliforms, particularly E. coli and V. parahaemolyticus, in seawater.
Methods
Experiments were conducted to evaluate the survival of various bacteria in seawater in the presence and absence of microbubblers. Equal volumes of seawater were added to four reaction chambers, each with a different water aeration and/or circulation system in place. Known concentrations of bacterial suspensions were added to the chambers. Bacterial concentrations were monitored for survival by sampling at appropriate intervals after inoculation and after treatment with the aeration/ circulation systems began.
Microbubblers
Two types of microbubblers were tested; a Canadian-manufactured all-plastic microbubbler (Island Scallops, Ltd, BC) and a similar Japanese manufactured device (Mori Engineering Works, Ltd, Japan) made of plastic but containing an internal baffle made of a metal alloy and a metal junction for the sipper tube. The research team who previously observed decreased bacterial counts in the presence of a microbubbler used the Japanese type which contained metallic parts. Other than the metallic parts, the devices were similar (Figure 1.)
Both tubes were approximately 20 cm long and function by the Venturi principle, whereby the movement of water pumped through the main body causes the drawing in of air via a "sipper" tube, thus generating small bubbles at the outlet.

Figure 1
Microbubbler devices used were: all-plastic (top), and plastic and metal (bottom). They differ largely in the presence of a metal baffle in the interior of the plastic and metal type, which was not present in the all-plastic device. The 'sipper' on the top of the device draws air in (through an airhose, not shown), by a Venturi effect, as water is pumped through the main chamber of the device.
Seawater Microcosms
The experimental chambers, each having a total volume of 26 L, were made of clear plastic and had tight-fitting, removable lids (Figure 2). External pumps were connected to the microbubblers and re-circulated 6 L of seawater within each of the chambers.
Four experimental set-ups were tested:
- The pump and the air intake of the all-plastic microbubbler working and producing micro-bubbles;
- The pump and the air intake of the plastic/metal microbubbler working and producing micro-bubbles;
- The pump working but the air intake of the all-plastic microbubbler closed, so that water was being circulated through the systems but not aerated with microbubbles; and
- Conventional aquarium air stone spargers, fitted with an external aquarium pump, providing aeration.
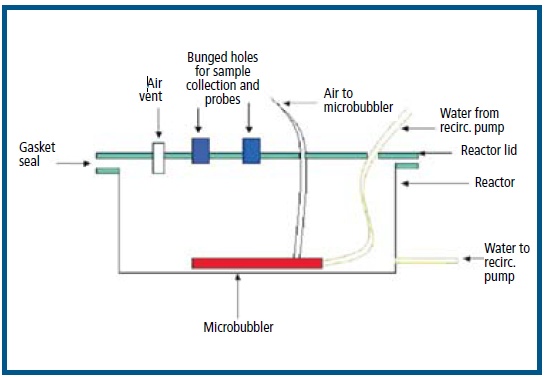
Figure 2
Schematic of microcosm reaction chamber showing major components.
Each experimental set-up was tested in triplicate, and all were monitored in comparison with control conditions in which a similar chamber was equipped with no water circulation or aeration devices.
Various physical, chemical, and biological factors can influence the survivability of bacteria. Seawater itself can negatively impact the viability of introduced organisms, such as E. coli and other fecal bacteria. Water temperature, salinity (of 26-32%), light, and pH levels were monitored and regulated for each seawater chamber throughout the experiment.
Precautions were taken to ensure that the microcosm chambers were not contaminated and would provide statistically valid comparisons of the effect of aeration with fine bubbles. All air intake lines and vents were fitted with sterile cotton wool to trap and prevent contamination from air-borne micro-organisms. Between experiments, chambers and all internal tubing were sanitized with bleach solution, rinsed with sterile, neutralized water, and then finally UV irradiated for 15 minutes.
Bacterial Culture and Enumeration
Pure cultures of E. coli and V. parahaemolyticus were purchased for the tests and these bacteria were cultivated aseptically in nutritional broths according to standard methods and were washed and re-suspended in filter-sterilized marine water before being added to the seawater chambers. In some experiments, slurries of fecal matter left by seals and seabirds on the wooden structures and floats in an oyster farm site were used as a source of naturally-occurring fecal bacteria.
E. coli and V. parahaemolyticus population concentrations in the seawater chambers were monitored by sampling over a period of 48 to 120 hours after inoculation and commencement of microbubble treatment. Epiflourescence microscopy and cultivation-based methods were used to determine bacterial survival.
Results
The experimental treatments using the all-plastic, microbubbler, the flow-only pump and all-plastic microbubbler, and the aquarium stones did not significantly affect the survivability of the E. coli populations over the first 48 hours. In an experiment carried out at 15 °C and with initial average bacterial counts of 1.0 x 107 cells per mL, approximately 1.8 x 106 cultivable cells per mL were recovered after 48 hours of aeration with an allplastic microbubbler. However, the treatment using the plastic/metal microbubbler resulted in a 5 logcycle decrease in cultivable bacteria within the first 24 hours, and an almost complete loss of cultivable bacteria after 48 hours (Figure 3). Similarly, a V. parahaemolyticus strain also showed a rapid decline in cultivability only when the plastic/metal microbubbler was used.
Seawater in long-term contact with the plastic/metal microbubbler was found to promote a more rapid decrease in cultivable bacteria than water from the other microcosms. It was then considered that some chemical component(s), detrimental to E. coli, was (were) either produced by, or leached from this type of microbubbler.
As many metals used in marine applications are copper-containing alloys, which in seawater have known antimicrobial effects, the possibility that copper may have leached from the microbubbler with metallic parts was tested. Detection of copper (Cu++) by a common aquarium test kit clearly showed that water in contact with the plastic/metal microbubbler accumulated increasing levels of copper over time, while no such accumulation was evident for all other devices or treatments (Table 1).
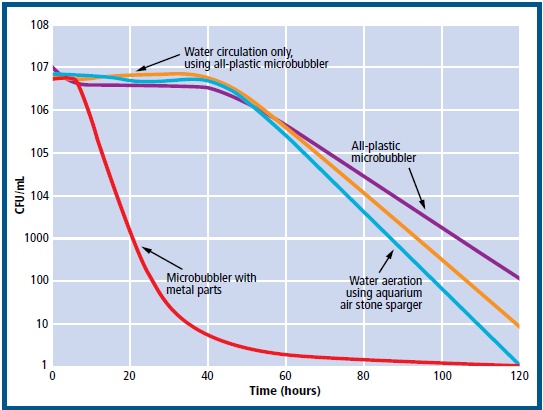
Figure 3
E. coli survivability trends (as colony-forming units (CFU) per mL of seawater) in seawater microcosms over a 120 hour period, for the 4 experimental set-ups examined. (When no cell growth was detected, a value of 1 CFU/mL was used for graphing purposes.)
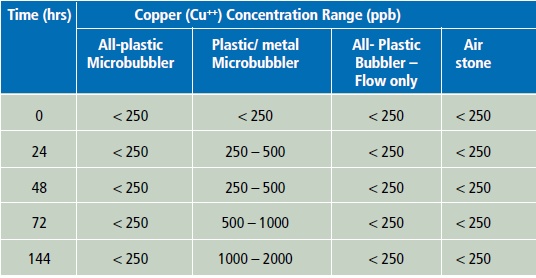
Table 1.
Copper (Cu++) levels in seawater microcosms exposed to bubbling or flow aeration with different devices over a period of 5 to 6 days.
Conclusions
Contrary to the indications of preliminary observations, low pressure aeration devices, "microbubblers", were not directly effective at lowering the survivability in seawater of the tested potential microbial contaminants of oysters.
It is well documented that copper from alloys dissolved in seawater has antimicrobial effects and, as such, copper is often used as an algicide and antifouling agent. It is suggested that copper leached from the metal components of the plastic/metal microbubbler and accumulated in the seawater to levels harmful to the microbes and that these high copper levels were responsible for the observed effects of lowered survivability of E. coli and V. parahaemolyticus in this and in previous studies.
While it has been determined that the use of "microbubblers" does not directly reduce the number of potential microbial contaminants in seawater, these devices may, however, be suitable as aeration devices in various oyster culture or other aquaculture applications, though this was not evaluated in this experiment.
This ACRDP project (P-06-01-001) was a collaborative effort among Fisheries and Oceans Canada (DFO Science), the British Columbia Shellfish Growers Association, Vancouver Island University (VIU), and VIU's Centre for Shellfish Research. The lead scientist on this project, John Amaral, can be contacted at John.Amaral@viu.ca.
For further information on this and other ACRDP projects, visit: /aquaculture/acrdp-pcrda/index-eng.htm.
Published by:
Aquaculture Science Branch
Fisheries and Oceans Canada
Ottawa, Ontario K1A 0E6
©Her Majesty the Queen in Right of Canada 2010
ISSN 1919-6806 (Print),
ISSN 1919-6814 (Online)
DFO/2008-1493
French version and alternative formats available through: http://www.dfo-mpo.gc.ca/acrdp-pcrda/index-fra.htm.
- Date modified: