Marteilia spp. of Mussels
On this page
Category
Category 1 (Not Reported in Canada)
Common, generally accepted names of the organism or disease agent
Marteiliosis of mussels.
Scientific name or taxonomic affiliation
Marteilia refringens, Marteilia maurini and Marteilia pararefringens currently occur in the phylum Cercozoa and order Paramyxida (Cavalier-Smith and Chao 2003, Feist et al. 2009, Alfjorden et al. 2017), although these parasites were assigned to the phylum Paramyxea in the past (Berthe et al. 2000, 2004). Marteilia refringens was initially described from Ostrea edulis (Grizel et al. 1974) and subsequently M. refringens was reported in 2% of the Mytilus edulis in Brittany, France (Comps et al. 1975). Comps et al. (1981 (1982), 1982) described M. maurini in Mytilus galloprovincialis from Venice lagoon, Adriatic Sea, Italy and indicated that its structural characteristics and developmental cycle were similar to that of M. refringens. Unfortunately no morphological features are available to differentiate between M. refringens and M. maurini (Villalba et al. 1993a, Longshaw et al. 2001). However, Le Roux et al. (2001) identified genetic dimorphism in the internal transcribed spacer region (ITS1) of the ribosomal RNA genes and supported the recognition of two species of Marteilia in Europe and proposed that the ''O'' type corresponds to M. refringens in oysters (O. edulis) and the ''M'' type to M. maurini in mussels (M. edulis and M. galloprovincialis). Nevertheless, the occurrence of “O” type in mussels and “M” type in oysters and co-infections of oysters and mussels by both genetic types occurred but were rare in some areas (Le Roux et al. 2001, Novoa et al. 2005, Arzul et al. 2014) and common in others (Novoa et al. 2005, Balseiro et al. 2007, Carrasco et al. 2015). From the results of molecular assays, López-Flores et al. (2004), Novoa et al. (2005) and Balseiro et al. (2007) suggested that the Marteilia from oysters and mussels may be two different strains of the same species that appear to readily infect both hosts in some locations. Arzul et al. (2014) and Carrasco et al. (2015, 2017) also supported the hypothesis that types M and O represent the same unique species, M. refringens. Balseiro et al. (2007) indicated that more research is required (e.g., into the molecular identity of new genes especially the coding genes, the relationship between the parasite and its different hosts, ecological studies to complete the life cycle, etc.) before the synonymy of the two species can be confirmed. The World Organisation for Animal Health (Office international des épizooties, OIE) Reference Laboratory for Infection with Marteilia refringens recognises two types of Marteilia refringens, types O and M (for oysters and mussels, respectively) as defined by Le Roux et al. (2001) (OIE 2012). Alfjorden et al. (2017) argued that M. refringens and M. maurini should conservatively be regarded as a single species, M. refringens, for most effective health management. However, Kerr et al. (2018) determined that there were at least two species of Marteilia in European mussels. Because it was not possible to determine the lineage nor genetic profile of M. maurini described by Comps et al. (1981 (1982)) from mussels in the Adriatic Sea, and because of the occurrence of several genotype variants related to M. refringens, Kerr et al. (2018) named the lineage that they typified from northern Europe M. edulis as Marteilia pararefringens (personal communications, Dr. David Bass, Pathology and Molecular Systematics, Cefas, Weymouth, UK). They also described a means of discriminating between M. refringens and M. pararefringens based upon a specific molecular diagnostic assay (Kerr et al. 2018).
Geographic distribution
The confusion over the specific identity of Marteilia detected in mussels throughout Europe creates challenges for reporting the distribution of the various isolates/species. Over all in Europe, infections with Marteilia spp. have been recorded in many mollusc species in an area extending along the Atlantic coasts of Europe from the Baltic Sea (Sweden and southern coast of England to Morocco, including Brittany (France), Galicia (Spain) and Portugal, and in the Mediterranean Sea (Le Roux et al. 2001, Berthe et al. 2004, Lopez-Flores et al. 2004, Novoa et al. 2005, Engelsma and Hine 2009, Carrasco et al. 2015, Guo and Ford 2016, Alfjorden et al. 2017). Following are further details and references pertaining to the geographic distribution of Marteilia spp. in mussels sorted according to host species, parasite identity and location from north to south in the Atlantic and west to east in the Mediterranean Sea.
In Mytilus edulis, the parasite named M. refringens has been reported from the Baltic Sea, Sweden (OIE 2010, report reference 8982 in Mytilus edulis 25/01/2012, Sweden), coastal Brittany, France (Comps et al. 1975, Tige and Rabouin 1976, Mialhe et al. 1985, Berthe et al. 2000), and Ria de Arousa, Spain (Gutierrez 1977, Perez Camacho et al. 1997). The parasite named as M. maurini was reported in M. edulis from northern Brittany (Auffret and Poder 1983 (1985)). Also in M. edulis, Marteilia sp. was isolated by Robledo et al. (1994a) and a Marteilia-like parasite was detected in China (Wang et al. 2012). Marteilia pararefringens was described in M. edulis from Bømlo, west Norway; northwest coast of Sweden; and the Tamar Estuary, southern England, UK (Kerr et al. 2018). Bayesian phylogeny analysis of the sequence of the full length rDNA gene (i.e., complete 18S-ITS1-5.8S-ITS2-28S-IGS ribosomal gene) grouped the sequences of Marteilia refringens (type M) in M. edulis from the UK and France with that of M. pararefringens (Kerr et al. 2018).
In Mytilus galloprovincialis, the parasite named as M. refringens has been reported from Galicia, Spain (Villalba et al. 1993a, b, 1997; Robledo et al. 1994b, 1995a; Robledo and Figueras 1995; Fuentes et al. 1995, 2002; Perez Camacho et al. 1997; Carballal et al. 1998; Darriba Couñago 2017) and on the Mediterranean coasts of Spain, Delta de l'Ebre (Carrasco et al. 2007b, 2008c), and France, Thau Lagoon (Boyer et al. 2013). The parasite identified as M. refringens type M was reported from the west coast of Spain (López-Flores et al. 2004, Novoa et al. 2005) and on the Mediterranean coasts of Spain, Delta de l'Ebre (Carrasco et al. 2017); Italy, west coast (Balseiro et al. 2007, Carella et al. 2010) and the Adriatic Sea (Gombač et al. 2013, Arzul et al. 2014). The parasite named as M. maurini was reported in M. galloprovincialis from the Adriatic Sea (Comps et al. 1981 (1982), 1982; Ceschia et al. 1992) and as “possibly M. maurini”, or “M. maurini (or type M)” from Galicia, Spain (Figueras et al. 1991, Figueras and Robledo 1993), and Delta de l'Ebre, Spain (Carrasco et al. 2007a, 2008b). Marteilia refringens / maurini was reported from M. galloprovincialis from the Gulf of Thermaikos, Aegean Sea, northern Greece (Rayyan et al. 2006). Unidentified Marteilia sp. were reported from Galicia, Spain (Pernas et al. 2000), south coast of Italy (Tiscar et al. 1993), Adriatic Sea (Zrnčić et al. 2001, Pellumb et al. 2006), and the Aegean Sea (Virvilis et al. 2003, Karagiannis and Angelidis 2007, Anestis et al. 2010). Bayesian phylogeny and/or Maximum Likelihood phylogeny analyses of the sequence of the full length rDNA gene (i.e., complete 18S-ITS1-5.8S-ITS2-28S-IGS ribosomal gene) grouped the sequences of Marteilia refringens (type M) in M. galloprovincialis from France, Spain and Italy with that of M. pararefringens (Kerr et al. 2018).
Wang et al. (2012) reported a Marteilia-like organism in the digestive gland of 2.8% of the Mytilus edulis from along the east coast of China. The genetic sequence of the 641 base pair product resulting from PCR amplification of a fragment of the 18S ribosomal RNA gene of this parasite using primers described by Le Roux et al. (1999) was 88% similar to that of Marteilia refringens (Wang et al. 2012) and is likely to be different from Marteilia spp. found in the mussels of Europe (Carrasco et al. 2015).
Host species
Marteilia maurini was originally described in Mytilus galloprovincialis (Comps et al. 1981 (1982), 1982) and later reported in Mytilus edulis (Auffret and Poder 1983 (1985)), but as indicated above, the validity of this species is now questioned. Nevertheless, in addition to mussels, M. refringens / maurini / pararefringens and Marteilia sp. were reported from oysters, possibly clams and cockles, scallops and other bivalves (Berthe et al. 2004, Engelsma and Hine 2009, Carrasco et al. 2015, Guo and Ford 2016, Alfjorden et al. 2017). Also reported in the black pigmy mussel, Xenostrobus securis, a non-indigenous invasive species in the Ría de Vigo, Galicia, Spain where it cohabits with infected M. galloprovincialis (Pascual et al. 2010). Auffret and Poder (1983 (1985)) mentioned an unpublished report in the horse mussel Modiolus modiolus on the Atlantic coast of Europe. Marteilia pararefringens was described from Mytilus edulis and also was detected in Ostrea edulis (Kerr et al. 2018).
Impact on the host
This parasite is considered to be a potentially lethal pathogen but mussels are usually not adversely affected by marteiliosis. For example, among 1280 cultured and wild adult Mytilus galloprovincialis, collected over a 1-year surveillance period from the Slovene Adriatic Sea, 0.3% were infected with only Marteilia refringens type M and only sporadic disruption of epithelial cells of digestive tubules and focal destruction of digestive tubules were observed (Gombeč et al. 2014). Tige and Rabouin (1976) detected M. refringens in only 1.4% of 703 M. edulis from the mouth of the Penzé River, Brittany, France where 80 to 100% of the flat oysters were infected. Auffret and Poder (1983 (1985)) reported that the effects of Marteilia maurini on M. edulis was variable but may be inconspicuous. Robledo and Figueras (1995) suggested that the mortality rate in M. galloprovincialis in comparison to the prevalence of infection could be explained by an elimination of the parasite by the mussel defense mechanisms rather than by death of infected mussels. In England, Norway and Sweden, no significant mortalities of mussels have occurred despite an overall prevalence of about 9% of M. pararefringens in these countries (Kerr et al. 2018). Berthe (2002) indicated that mussels (M. edulis and M. galloprovincialis) from enzootic areas were susceptible to infection but not affected by (tolerant to) M. refringens / maurini, possibly because mussels, with a long historical association with M. refringens, found an equilibrium host–parasite relationship, which helped to maintain the parasite prevalence at low levels and the associated mortalities reduced or null. Because mussels tend to experience relatively low disease levels of this parasite in comparison to oysters, Guo and Ford (2016) suggested the possibility that mussels might act as an alternative/reservoir host for the oyster parasite in areas where both are cultured. This suggestion is contrary to the experimental results of Figueras and Robledo (1993) who did not detect any transmission between infected Mytilus galloprovincialis and uninfected Ostrea edulis farmed for two years on the same raft in Ría de Vigo, Galicia, Spain. Whether or not mussels play a role in the transmission of the parasite to oysters has yet to be established (ICES 2012).
However, in some areas, mortality attributed to this parasite is significant for the mussel farming industry. Berthe et al. (2004) reported that naive M. edulis originating from an area free of Marteilia spp. experienced mass mortalities when transferred to the enzootic area. For example, mussel mortalities (up to 100%) associated with heavy infection by M. refringens / maurini were reported in the past in France in M. edulis bought in from Northern European countries for relaying in France. These mussels had no previous contact with M. refringens / maurini and were possibly highly susceptibility to the disease (Arzul 2011, Carrasco et al. 2015). Also, Fuentes et al. (2002) reported that hybrid crosses between M. edulis and M. galloprovincialis were more heavily parasitised than three M. galloprovincialis stocks cultured for one year under a commercial raft in northwest Spain. The average prevalence of infection in M. galloprovincialis from five rías in Galicia, NW Spain ranged up to 35% between 1985 and 1989 (Figueras et al. 1991, Villalba et al. 1997). Virvilis et al. (2003) suggested that the increased mortality of M. galloprovincialis during the summer may be attributed to Marteilia sp. in the Gulf of Thermaikos, northern Greece.
Figueras et al. (1991), Villalba et al. (1997), Rayyan et al. (2006) and Anestis et al. (2010) noted that infection of M. refringens / maurini in mussels was associated with haemocyte infiltration of the digestive gland (connective tissue and epithelia) and extensive destruction of the digestive gland occurred in heavy infections. Pathologic disruption of the digestive gland tubule epithelia was most severe at the time of spore release from the sporangia (Robledo and Figueras 1995). Heavy infections also caused reduction of absorption efficiency resulting in an inhibition of gonad and storage tissue development and thus a significant loss in condition of infected mussels (Villalba et al. 1993b, Robledo et al. 1995a, Pérez Camacho et al. 1997, Anestis et al. 2010). Infected M. galloprovincialis also had lower total carbohydrate concentrations in the haemolymph (Robledo et al. 1995a) and a significant increase in circulating haemocytes, especially hyalinocytes, (Carballal et al. 1998) than uninfected mussels. The infection usually triggers a haemocytic reaction that may slow or even stop the infection at times. Association between mussel mortality and this parasite has been suggested in some locations (Villalba et al. 1993a). Anestis et al. (2010) determined that compared to non-infected M. galloprovincialis, the Scope for Growth (SFG) values of infected mussels was significantly lower (P ˂ 0.05), with the degree of SFG reduction dependent on the intensity levels of infection by Marteilia sp.. These differences were attributed to the higher filtration rate and the lower absorption efficiency detected in the infected mussels (Anestis et al. 2010). In China, Wang et al. (2012) reported infected M. edulis were emaciated. However, further examination is needed to more fully understand the parasite's pathological effect on the host in China (Carrasco et al. 2015).
The complete life cycle of Marteilia refringens/maurini has not been fully described (Engelsma and Hine 2009). Although Comps and Joly (1980) were able to infect mussels (Mytilus galloprovincialis) with M. refringens extracted from oysters (Ostrea edulis), Berthe et al. (1998, 2004) supported the hypothesis that intermediate or alternate hosts (unknown) or free-living stages (also unknown) were essential in the life cycle of this parasite. Research into potential intermediate hosts in French shallow-water oyster ponds ('claires'), where species diversity is relatively limited, detected M. refringens DNA in various invertebrates with the most promising possibility being the Calanoida copepod Paracartia (= Acartia) grani (Audemard et al. 2001, 2002). Boyer et al. (2013) supported the hypothesis of the transmission of M. refringens from M. galloprovincialis to P. grani and Arzul et al. (2014) detected M. refringens (by in situ hybridization) in the gonadal tissue of female Paracartia latisetosa from Diana Lagoon, Corsica after the peak of prevalence in mussels (M. galloprovincialis).
Although P. grani could be infected with M. refringens / maurini from both oysters (O. edulis) and mussels (M. galloprovincialis), attempts to transmit M. refringens from P. grani to O. edulis and mussels (Mytilus edulis) were unsuccessful (Audemard et al. 2002, Carrasco et al. 2008a). Also, Carrasco et al. (2008a) reported that the infection patterns of M. refringens / maurini in P. grani were different for copepods infected via M. galloprovincialis or via O. edulis with only early stages of infection found in the intestinal tract of P. grani infected from mussels compared to higher prevalence and intensity of infections in the intestinal tract and gonad of P. grani infected from oysters. Nevertheless, Boyer et al. (2013) observed unusual M. refringens cells in the digestive tract and the gonad from the third copepodid stage of P. grani and suggested that the parasite could infect a copepod by ingestion and be released through the gonad. This hypothesis was supported by the PCR detection of parasite DNA in copepod eggs from PCR-positive females, suggesting that eggs of P. grani could contribute to the parasite spreading in the water and could allow overwintering of M. refringens. Also, experimental results showed that all copepod stages of P. grani could contribute to the transmission of M. refringens, especially eggs and nauplii, because they were retained by up to 90% by M. galloprovincialis (Boyer et al. 2013).
Carrasco et al. (2007a, b, 2008b) detected M. refringens DNA in other copepods (3 Calanoida, Acartia discaudata, A. clausi and A. italica; 1 Cyclopoida, Oithona sp.; and at least 1 Harpacticoida, Euterpina acutifrons and an unidentified Harpaticoida species) and in larval stages of decapod crustaceans (zoea larvae of Brachyura, probably Portumnus sp.) from the natural bays of the Ebro (Ebre) Delta (NW Mediterranean Sea, Spain) where mussels are the predominate farmed mollusc. Burreson (2008) indicated that the PCR assay used by Carrasco et al. (2007a, b) was not validated to detect Marteilia spp. Because the PCR-only results from unvalidated assays were not confirmed by histology, they should be interpreted with caution and the involvement of these organisms in the life cycle of M. refringens / maurini remains unknown.
The developmental stages of Marteilia spp. in bivalves were described by Grizel et al. (1974), Perkins (1976), and Kleeman et al. (2002a), and summarized by Bower (2006) as follows. Infections by all Marteilia spp. are presumably initiated by a primary cell or stem cell (5 to 8 μm in diameter) in the epithelial cells of the gut or gills. Carrasco et al. (2008c) detected initial (early) infections in the gill and mantle epithelium of M. galloprovincialis using the in situ hybridisation technique. The primary uninucleate cell develops a secondary uninucleate daughter cell in a vacuole within its cytoplasm. The daughter cell divides by binary fission to produce four daughter cells within the enlarged primary (stem) cell and within each daughter cell a uninucleate cell develops by internal cleavage. The primary cell degenerates to release the daughter cells, which become new primary cells. In the gut, the parasite penetrates the basal membrane of the digestive gland tubules and becomes established as nurse cells at the base of the epithelial cells. Nurse cells containing daughter cells proliferated and eventually degraded. Daughter cells in the digestive gland tubules become sporangiosori called “primary cells” by Perkins and Wolf (1976) and pansporoblasts by Mialhe et al. (1985). Sporulation occurs within the sporangiosorus via a unique process of internal cleavages (endosporulation) to produce cells within cells (Fig. 1). At the initiation of sporulation, uninucleate segments become delimited within the cytoplasm of the sporangiosorus to form the sporangial primordia (secondary cells). Eventually, 8 to 16 sporangial primordia (each about 12 μm in diameter at maturity) form within the sporangiosorus that retains its nucleus and enlarges to about 30 μm in diameter. Each sporangial primordium matures into a sporont containing 2 to 4 spore primordia (tertiary cells) that mature into spores (Fig. 1). Each spore contains 3 uninucleate sporoplasms of graded sizes, with each of the smaller sporoplasms being enclosed within the cytoplasm of the next largest one (i.e., consecutive internal cleavage of two sporoplasms within the spore primordium) (Perkins 1976). A continuous spore wall with no operculum occurs around each spheroid mature spore that measures 3.5 to 4.5 μm in diameter. As the spore matures, light refractile inclusion bodies appear in the sporont cytoplasm surrounding the spores. The specific name of M. refringens was derived from these 'refringent' inclusion bodies. Mature spores are shed into the tubule lumen for evacuation from the mussel and infected mussels may shed large numbers of spores before death.
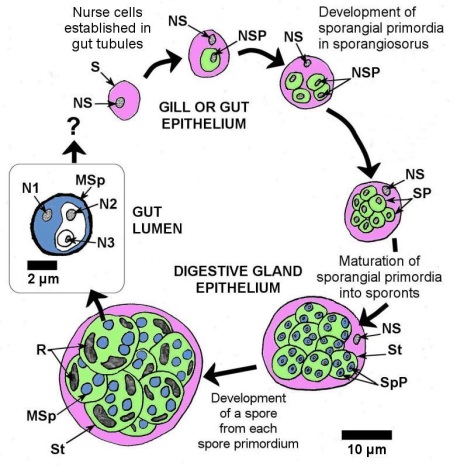
Figure 1. Schematic drawing to scale, of the sporulation process of Marteilia spp. with the cytoplasm of each stage colour coded for easy recognition. S = sporangiosorus (or primary cell, pink coloured cytoplasm), NS = nucleus of sporangiosorus, SP = sporangial primordium that matures into a sporont (the secondary cell, green cytoplasm), NSP = nucleus of sporangial primordium, St = sporont, SpP = spore primordium that matures into the spore (the tertiary cell, blue cytoplasm), MSp = mature spore, R = refringent bodies, N1 = nucleus of outer most sporoplasm, N2 = nucleus of middle sporoplasm, N3 = nucleus of inner most sporoplasm.
The seasonal pattern of the infection in mussels was different between the sites studied. For example, in Galicia and Catalonia, Spain, higher prevalences of Marteilia spp. in M. galloprovincialis were observed in July, August and September with sporulation occurring throughout the year (Villalba et al. 1993a, Carrasco et al. 2007b). In Thau lagoon, France, sporangial forms of M. refringens in mussels was bimodal with peaks in spring (April, May) and autumn (September, October) when temperatures ranged from 15.0 to 20.5° C suggesting that in Thau lagoon, the parasite has two cycles per year and that mussels release parasites into the water column during these two periods (Boyer et al. 2013).
Diagnostic techniques
Gross Observations
Clinical signs include dead or gaping molluscs (2 or more years old), especially when temperature is at a maximum for mussels (Carrasco et al. 2007b; OIE 2012). Reduced growth rate and inhibition of gonad development were reported for infected mussels (Villalba et al. 1993b). However, these clinical signs are not specific to infection with M. refringens / maurini and could be indicative of other infections (Berthe et al. 2004).
Wet mounts
In advanced infection, mature sporangia with refringent granules can be observed in wet mounts from gaping mussels, freshly dead mussels or faeces of live diseased mussels. Squash a piece of digestive gland or faeces from suspect mussels on a glass slide. Observations are then made at ×400 magnification and can potentially show refringent granules in mature sporangia. A positive result is the presence of large (20–30 μm) spherical bodies containing spherical thick walled structures (spores). In susceptible species, within the known geographic range of infection with M. refringens / maurini, a positive result is indicative of infection with this parasite. In other species, or outside the known geographic range of infection with M. refringens / maurini, a positive result is indicative of infection with a Marteilia species that needs to be confirmed by the OIE Reference Laboratory (OIE 2012).
Smears/Tissue Imprints
In advanced infection, parasites ranging in size up to 30–40 μm can be observed in digestive gland imprints from gaping mussels or freshly dead mussels (Carella et al. 2010). Prepare digestive gland imprints of suspect mussels by drying excised tissues on absorbent paper and make several imprints on a glass slide. Air-dried slides are then fixed in methanol or in absolute ethanol and stained using a commercially available blood-staining kit, in accordance with the manufacturer's instructions (e.g., Hemacolor, Merck; Diff-QuiK, Baxter, etc.). After rinsing in tap water and drying, the slides are mounted with a cover-slip using an appropriate synthetic resin. Slides are observed first at ×200 magnification and then under oil immersion at ×1000 magnification. Note that because infections may be focal and because the early and late stages of infection targets different tissues, imprints might miss early and low levels of infection. A positive result is the observation of cells ranging in size up to 30–40 μm in diameter with basophilic cytoplasm, eosinophilic nucleus, pale halos around large, strongly stained (refringent) granules and, in larger cells, cell within cell arrangements may be evident (Grizel et al. 1974, Berthe et al. 2000, Berthe et al. 2004, for colour image see Arzul 2011). In susceptible species, within the known geographic range of infection with M. refringens / maurini, a positive result is strongly indicative of infection with this parasite. In other species, or outside the known geographic range of infection with M. refringens / maurini, a positive result is indicative of infection with a Marteilia species that needs to be confirmed by the OIE Reference Laboratory (OIE 2012).
Histology
Various stages of the parasite (as described above) can be observed in the epithelial cells of the digestive gland ducts (basophilic stages, mainly nurse cells (plasmodia)) and the epithelial cells of the digestive tubules (acidophilic stages, mainly sporangiosori at various stages of development) (Carella et al. 2010, Darriba Couñago 2017). Parasites in the major ducts and stomach (mean 9.9 µm in diameter with internal primary cells about 4.1 µm in longest axis) are slightly larger than those in primary and secondary digestive tubules (mean 8.4 µm in diameter). The sporangiosori, containing eight sporangial primordia (sporont), enlarge up to 19.6 µm in diameter and each sporangial primordia eventually contains 3-4 spores (mean 2.6 µm in diameter). Refringent granules appear during the course of sporulation and can range from deep orange to deep red in colour in tissues stained with haematoxylin and eosin stain.

Figure 2. Sporangiosori at different stages of development (arrows) and developing spores (arrowheads) of Marteilia maurini / refringens in digestive gland tubule epithelium of Mytilus edulis. Image from histological material supplied by I. Arzul of the OIE reference laboratory for Marteilia refringens. Haematoxylin and eosin stain.
Electron Microscopy
Only the outer sporoplasm of the spore contains haplosporosomes (130-400 × 130-200 nm). Comps et al. (1981) indicated that M. refringens could be differentiated from Marteilia maurini in Mytilus galloprovincialis by subtle differences in haplosporosome shape and the “existence of a multimembraneous envelope next to the spore wall”. However, Auffret and Poder (1983) and Longshaw et al. (2001) concluded that these criteria are invalid. Haplosporosomes in mature Marteilia from oysters and mussels were similar in shape (sphaeroid and oblate), although those from mussels were marginally smaller in size. Spore wall morphology was found to vary depending on the state of maturity of the parasite. Nevertheless, ultrastructural criteria are not sufficient to discriminate between Marteilia refringens and M. maurini (Balseiro et al. 2007, Arzul 2011).
Isolation and Purification
Mialhe et al. (1985) described a procedure for the isolation and purification of Marteilia sp. from oysters and mussels by the application of density gradients to a homogenate of heavily infected digestive gland. Further information on the isolation of various developmental stages from the digestive gland of M. galloprovincialis were detailed by Robledo et al. (1995b).
Immunological Assay
Tiscar et al. (1993) prepared polyclonal antibodies to Marteilia sp. isolated from Mytilus galloprovincialis from southern Italy that was used to clearly mark the parasite by direct immunoperoxidase staining in smears of the digestive gland of infected mussels. Monoclonal antibodies from six clones obtained from mice (Balb/c) against Marteilia sp. from Mytilus edulis in Brittany, France were specific for Marteilia spp. and cross reacted with Marteilia refringens from Mytilus galloprovincialis in Ría de Vigo, Spain. Four of the monoclonal antibodies reacted with the spore wall and two with the spore cytoplasm (Robledo et al. 1994a). These monoclonal antibodies were found to produce various results when used in different immunological tests (Pernas et al. 2000).
DNA Probes
The nucleotide sequence of the small subunit ribosomal RNA (SSU rDNA or 18S rDNA) gene was compared with that of various eukaryotic organisms and polymerase chain reaction (PCR) primers were designed (Le Roux et al. 1999). The specificity of the amplified fragment for Marteilia sp. was confirmed by Southern blotting with an oligoprobe. Berthe et al. (2000) reported that the SSU rDNA gene sequence of Marteilia sp. that they isolated from O. edulis and M. edulis collected in different location in France were identical. Therefore, the World Organisation for Animal Health recommends PCR primers that target the internal transcribed spacer 1 (ITS1) region described by Le Roux et al. (2001) to amplify M. refringens (OIE 2012). Le Roux et al. (2001) and other investigators (Novoa et al. 2005, Balseiro et al. 2007) subjected the PCR product to restriction fragment length polymorphism (RFLP) with the endonuclease HhaI to differentiate between the O and M types of Marteilia sp. isolates. Balseiro et al. (2007) used this PCR-RFLP to analyse Marteilia sp. isolates from oysters and mussels from various locations in Europe to support the synonymy of M. maurini with M. refringens. In Marteilia from mussel and oyster sampled from the Atlantic and Mediterranean coasts of Spain, Novoa et al. (2005) detected O type in M. galloprovincialis and M types in O. edulis and used estimated phylogeny to indicate that some 'O' types had switched to 'M' types and vice versa. Gombač et al. (2013) stressed the need for sequencing to complement the established PCR-RFLP analysis for correct parasite typing. Carrasco et al. (2017) developed a competitive real-time PCR assay based on the ITS1 of M. refringens for rapid and sensitive detection of M. refringens which discriminated between “M” and “O” genotypes of M. refringens as well as the closely related M. cochillia. This real-time PCR assay was shown to be analytically sensitive and specific and has a high repeatability and efficiency (Carrasco et al. 2017).
In addition to ITS1 region analysis, a nested PCR assay (that has been tested only with M. refringens from O. edulis from Huelva, SW Spain and from M. galloprovincialis also from Huelva, SW Spain and two locations in NW Italy) was developed using primers targeting the rDNA intergene spacer IGS (López-Flores et al. 2004). This assay was demonstrated to be more sensitive than ITS1 PCR assay but needs to be tested more thoroughly for specificity (OIE 2012). Kerr et al. (2018) described nested diagnostic PCR primers that amplify a 1034 bp region from the 3′end of the 18S rRNA gene V9 region through to the 5.8S rRNA gene, which spans the whole of ITS1. The sequence of regions of the product were used to differentiate between M. refringens and M. pararefringens (Kerr et al. 2018). As infection may be focal and also because infection targets different tissues in the early and late stages of parasite development, the sensitivity of PCR detection may be lower than the expected theoretical PCR performance. A nested PCR reaction using probes from unknown locations in the genome was developed for the diagnosis of M. refringens in Mytilus galloprovincialis from Galicia, Spain and this test was more sensitive in detecting Marteilia in 20 mussels than smears and histology (Pernas et al. 2001). Sequences that present regions of the rDNA are available in the public gene banks.
In situ hybridisation (ISH) protocols have been developed and published (Le Roux et al. 1999, Berthe et al. 2000). Probes that targets the SSU of the rRNA gene complex and have been validated against histology (Le Roux et al. 1999, Thébault et al. 2005) are recommended by the World Organisation for Animal Health (OIE 2012). However, the probe, named Smart 2, was shown to cross react with Marteilia sydneyi and Marteilioïdes chungmuensis (Kleeman et al. 2002b). Nevertheless, Zrnčić et al. (2001) used this probe to detect Marteillia sp. in Mytilus galloprovincialis in Croatia and Carrasco et al. (2008c) used it to detect the initial infective stages of M. refringens in the epithelium of the gills, mantle, stomach and primary digestive tubules of M. galloprovincialis. In addition, an ISH assay was developed using a probe targeting the rDNA intergene spacer (IGS) (López-Flores 2008a, 2008b). This assay seemed to be more specific than the SSU ISH assay but needs to be thoroughly validated. In situ hybridization can help to detect early infections which are more difficult to detect in traditional histological sections (Arzul 2011).
Methods of control
Do not transfer mussels from areas known to be infected (currently or historically) to areas with no record of M. refringens / maurini unless an appropriate analysis is performed to estimate the risk of introduction of the parasite including aspects of cultural practices and knowledge of the parasite life cycle (Balseiro et al. 2007). Mussels from areas where Ostrea edulis is known to carry Marteilia refringens should be treated with similar caution. Carella et al. (2010) suggested that M. refringens type M may have been imported into the Campanian coast (Tirrenian Sea, south Italy) with recent transplantation of M. galloprovincialis seed.
Aquaculture practices in various areas have identified various procedures to address the issue of marteiliosis in mussels. For example, mussels from the inner part of two rías in Galicia, Spain, and those held at less depth (2 m rather than 5 m) in one ría had higher mean prevalence of infection. Thus, culture rafts located in the outer zones of the rías contribute to minimizing the impact of this parasite on the mussel culture industry in Galicia (Fuentes et al. 1995, Villalba et al. 1997). In the Thermaikos Gulf, the prevalence of Marteilia sp. was significantly greater in mussels cultured on tables than on long-lines (Karagiannis and Angelidis 2007). Also, water temperatures above 25 ºC for extended periods was detrimental to Mytilus galloprovincialis especially when infected by Marteilia sp. (Anestis et al. 2010). Robledo et al. (1994b) suggested that the collection of mussel seed from areas free of Marteilia sp. may contribute to a reduction in the prevalence of the parasite in cultured stocks.
References
- Alfjorden, A., M. Areskog, D. Bruno, R. Carnegie, D. Cheslett, S. Feist, S. Ford, S. Jones, A. Lillehaug, L. Madsen, T. Renault, N. Ruane and P. Vennerström. 2017. New Trends in Important Diseases Affecting the Culture of Fish and Molluscs in the ICES Area 2002 – 2015. In: Anderson, E.D., N. Ruane, R. Carnegie (eds.) ICES Cooperative Research Report No. 337, International Council for the Exploration of the Sea, Conseil International pour l'Exploration de la Mer, Copenhagen, Denmark. 50 pp.
- Anestis, A., H.O. Pörtner, D. Karagiannis, P. Angelidis, A. Staikou and B. Michaelidis. 2010. Response of Mytilus galloprovincialis (L.) to increasing seawater temperature and to marteliosis: Metabolic and physiological parameters. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 156: 57-66.
- Arzul, I. 2011. EURL (European Union Reference Laboratory) for Molluscs Diseases: Marteilia refringens. Web page hosted by Ifremer.
- Arzul, I., B. Chollet, S. Boyer, D. Bonnet, J. Gaillard, Y. Baldi, M. Robert, J.-P. Joly, C. Garcia and M. Bouchoucha. 2014. Contribution to the understanding of the cycle of the protozoan parasite Marteilia refringens. Parasitology 141: 227-240.
- Audemard, C., A. Barnaud, C.M. Collins, F. Le Roux, P.-G. Sauriau, C. Coustau, P. Blachier and F.C.J. Berthe. 2001. Claire ponds as an experimental model for Marteilia refringens life-cycle studies: new perspectives. Journal of Experimental Marine Biology and Ecology 257: 87-108.
- Audemard, C., F. Le Roux, A. Barnaud, C. Collins, B. Sautour, P.-G. Sauriau, X. De Montaudouin, C. Coustau, C. Combes and F. Berthe. 2002. Needle in a haystack: involvement of the copepod Paracartia grani in the life-cycle of the oyster pathogen Marteilia refringens. Parasitology 124: 315-323.
- Auffret, M. and M. Poder. 1983 (1985). Recherches sur Marteilia maurini, parasite de Mytilus edulis sur les côtes de Bretagne nord. (Studies on Marteilia maurini, parasite of Mytilus edulis from the north coasts of Brittany). Revue des Travaux de l'Institut des Pêches Maritimes. 47: 105-109. (In French with English abstract).
- Balseiro, P., A. Montes, G. Ceschia, C. Gestal, B. Novoa and A. Figueras. 2007. Molecular epizootiology of the European Marteilia spp., infecting mussels (Mytilus galloprovincialis and M. edulis) and oysters (Ostrea edulis): an update. Bulletin of the European Association of Fish Pathologists 27: 148-156.
- Berthe, F.C.J. 2002. Pacem in terris pathogenibus bonae voluntatis: mollusc-pathogens relationships prospects. Bulletin of the European Association of Fish Pathologists 22: 52-57.
- Berthe, F.C., M. Pernas, M. Zerabib, P. Haffner, A. Thébault and A.J. Figueras. 1998. Experimental transmission of Marteilia refringens with special consideration of its life cycle. Diseases of Aquatic Organisms 34: 135-144.
- Berthe, F.C.J., F. Le Roux, E. Peyretaillade, P. Peyret, D. Rodriguez, M. Gouy and C.P. Vivarès. 2000. Phylogenetic analysis of the small subunit ribosomal RNA of Marteilia refringens validates the existence of Phylum Paramyxea (Desportes and Perkins 1990). The Journal of Eukaryotic Microbiology 47: 288-293.
- Berthe, F.C.J., F. Le Roux, R.D. Adlard and A. Figueras. 2004. Marteiliosis in molluscs: A review. Aquatic Living Resources 17: 433-448.
- Bower, S.M. 1992. Diseases and parasites in mussels. In: E. Gosling (ed.) The Mussel Mytilus: Ecology, Physiology, Genetics and Culture. Elsevier Press, Amsterdam, p. 543-563.
- Bower, S.M. 2006. Parasitic diseases of shellfish. In: Woo, P.T.K. (ed.) Fish Diseases and Disorders, Volume 1: Protozoa and Metazoan Infections, Second Edition, CABI, Wallingford. pp. 629-677.
- Bower, S.M. and A.J. Figueras. 1989. Infectious diseases of mussels, especially pertaining to mussel transplantation. World Aquaculture 20(4): 89-93.
- Boyer, S., B. Chollet, D. Bonnet and I. Arzul. 2013. New evidence for the involvement of Paracartia grani (Copepoda, Calanoida) in the life cycle of Marteilia refringens (Paramyxea). International Journal for Parasitology 43: 1089-1099.
- Burreson, E.M. 2008. Misuse of PCR assay for diagnosis of mollusc protistan infections. Diseases of Aquatic Organisms 80: 81-83.
- Cavalier-Smith, T. and E.E.Y. Chao. 2003. Phylogeny and Classification of Phylum Cercozoa (Protozoa). Protist 154: 341-358.
- Carballal, M.J., A. Villalba and C. López. 1998. Seasonal variation and effects of age, food availability, size, gonad development, and parasitism on the hemogram of Mytilus edulis. Journal of Invertebrate Pathology 72: 304-312.
- Carella, F., S. Aceto, R. Marrone, P. Maiolino and G. De Vico. 2010. Marteilia refringens infection in cultured and natural beds of mussels (Mytilus galloprovincialis) along the Campanian coast (Tirrenian Sea, South of Italy). Bulletin of the European Association of Fish Pathologists 30: 189-196.
- Carrasco, N., I. López-Flores, M. Alcaraz, M.D. Furones, F.C.J. Berthe and I. Arzul. 2007a. First record of a Marteilia parasite (Paramyxea) in zooplankton populations from a natural estuarine environment. Aquaculture 269: 63-70.
- Carrasco, N., I. López-Flores, M. Alcaraz, M.D. Furones, F.C.J. Berthe and I. Arzul. 2007b. Dynamics of the parasite Marteilia refringens (Paramyxea) in Mytilus galloprovincialis and zooplankton populations in Alfacs Bay (Catalonia, Spain). Parasitology 134: 1541-1550.
- Carrasco, N., I. Arzul, B. Chollet, M. Robert, J.P. Joly, M.D. Furones and F.C.J. Berthe. 2008a. Comparative experimental infection of the copepod Paracartia grani with Marteilia refringens and Marteilia maurini. Journal of Fish Diseases 31: 497-504.
- Carrasco, N., I. Arzul, F.C.J. Berthe, M. Fernández-Tejedor, M. Durfort and M.D. Furones. 2008b. Delta de l'Ebre is a natural bay model for Marteilia spp. (Paramyxea) dynamics and life-cycle. Diseases of Aquatic Organisms 79: 65-73.
- Carrasco, N., I. Arzul, F.C.J. Berthe and M.D. Furones. 2008c. In situ hybridization detection of initial infective stages of Marteilia refringens (Paramyxea) in its host Mytilus galloprovincialis. Journal of Fish Diseases 31: 153-157.
- Carrasco, N., T.J. Green and N. Itoh. 2015. Marteilia spp. parasites in bivalves: A revision of recent studies. Journal of Invertebrate Pathology 131: 43-57.
- Carrasco, N., M. Voorbergen-Laarman, B. Lacuesta, D. Furones and M.Y. Engelsma. 2017. Application of a competitive real time PCR for detection of Marteilia refringens genotype “O” and “M” in two geographical locations: the Ebro Delta, Spain and the Rhine-Meuse Delta, the Netherlands. Journal of Invertebrate Pathology 149: 51-55.
- Ceschia, G., A. Mion, G. Orel and G. Giorgetti. 1992. Indagine parassitologica delle mitillicolture del Friuli-Venezia Giulia (Nord-Est Italia). Parasitological research in mussel cultures of Friuli-Venezia Giulia (North-East Italy). Bollettino Societa Italiana di Patologia Ittica 9: 24-36. (In Italian with English summary).
- Comps, M. and J.P. Joly. 1980. Contamination expérimentale de Mytilus galloprovincialis Lmk par Marteilia refringens. Science et Pêche Bulletin d'Information et de Documentation de l'Institut Scientifique et Technique des Pêches Maritimes 301: 19-21. (In French).
- Comps, M., H. Grizel, G. Tige‚ and J.L. Duthoit. 1975. Parasites nouveaux de la glande digestive des mollusques marins Mytilus edulis L. et Cardium edule L. (New parasites in the digestive gland of Mytilus edulis L. and Cardium edule L.). Comptes Rendus Académie des Sciences de Paris, Série D 281: 179-181. (In French).
- Comps, M., Y. Pichot and P. Papagianni. 1981 (1982). Recherche sur Marteilia maurini n. sp. parasite de la moule Mytilus galloprovincialis Lmk.(Research on Marteilia maurini n. sp. parasite of the mussel Mytilus galloprovincialis Lmk.). Revue des Travaux de l'Institut des Pêches Maritimes 45: 211-214. (In French, with English summary).
- Comps, M., H. Grizel and Y. Papayanni.1982. Infection parasitaire causée par Marteilia maurini sp. n. chez la moule Mytilus galloprovincialis International Council for Exploration of the Sea C.M. 1982/F: 24: 3 pp. (In French with English summary).
- Darriba Couñago, S. 2017. Atlas de Histopatoloxía, Moluscos bivalvos mariños. Histopathological Atlas, Marine bivalve molluscs. Published by Intecmar. Xunta de Galicia (Consellería do Mar), Edificios administrativos - San Caetano, s/n, Santiago de Compostela, Spain. (In Spanish and English).
- Engelsma, M and M. Hine. 2009. Infection with Marteilia refringens: disease detection, pathogen identification and typing. In: Hill, B., A. Reese, P. Dixon, B. Oidtmann, R. Paley, E. Peeler, G. Stentiford, D. Stone, K. Way, M. Hine, P. Calistri, C. Ippoliti, A. Di Lorenzo, L. Savini, O. Haenen and M. Engelsma. 2010. Epidemiology of different agents causing disease in aquatic animals: scientific review and database development (Parma, Italy, European Food Safety Authority (EFSA), 21 p.) Annex B, pp. 55-57.
- Feist, S.W., P.M. Hine, K.S. Bateman, G.D. Stentiford and M. Longshaw. 2009. Paramarteilia canceri sp. n. (Cercozoa) in the European edible crab (Cancer pagurus) with a proposal for the revision of the order Paramyxida Chatton 1911. Folia Parasitologica 56: 73-85.
- Figueras, A. and J.A.F. Robledo. 1993. Does the Marteilia present in mussels (Mytilus galloprovincialis) infect flat oysters (Ostrea edulis)? Bulletin of the European Association of Fish Pathologists 13: 97-99.
- Figueras, A.J., C.F. Jardon and J.R. Caldas. 1991. Diseases and parasites of rafted mussels (Mytilus galloprovincialis Lmk): preliminary results. Aquaculture 99: 17-33.
- Fuentes, J., A. Villalba, C. Zapata and G. Alvarez. 1995. Effects of stock and culture environment on infections by Marteilia refringens and Mytilicola intestinalis in the mussel Mytilus galloprovincialis cultured in Galicia (NW Spain). Diseases of Aquatic Organisms 21: 221-226.
- Fuentes, J., J.L. López, E. Mosquera, J. Vázquez, A. Villalba and G. Alvarez. 2002. Growth, mortality, pathological conditions and protein expression of Mytilus edulis and M. galloprovincialis crosses cultured in the Ría de Arousa (NW of Spain). Aquaculture 213: 233-251.
- Gombač, M., D. Kušar, M. Ocepek, M. Pogačnik, I. Arzul, Y. Couraleau and V. Jenčič. 2013. Marteiliosis in mussels: a rare disease? Journal of Fish Diseases 37: 805-814.
- Grizel, H., M. Comps, J.R. Bonami, F. Cousserans, J.L. Duthoit and M.A. LePennec. 1974. Research on the agent of digestive gland disease of Ostrea edulis Linné. Science et Pêche Bulletin d'Information et de Documentation de l'Institut Scientifique et Technique des Pêches Maritimes 240: 7-30. (In French).
- Guo, X. and S.E. Ford. 2016. Infectious diseases of marine molluscs and host responses as revealed by genomic tools. Philosophical Transactions of the Royal Society B: Biological Sciences 371: 20150206.
- Gutiérrez, M. 1977. Nota sobre marteiliasis en el mejillón, Mytilus edulis (L), de la costa Noroeste de España. Investigaciones pesquas 41: 637-642. (In Spanish, English summary).
- Karagiannis, D. and P. Angelidis. 2007. Infection of cultured mussels Mytilus galloprovincialis by the protozoan Marteilia sp. in the Thermaikos Gulf (N Greece). Bulletin of the European Association of Fish Pathologists 27: 131-141.
- Kerr, R., G.M. Ward, G.D. Stentiford, A. Alfjorden, S. Mortensen, J.P. Bignell, S.W. Feist, A. Villalba, M.J. Carballal, A. Cao, I. Arzul, D. Ryder and D. Bass. 2018. Marteilia refringens and Marteilia pararefringens sp. nov. are distinct parasites of bivalves and have different European distributions. Parasitology Published online: 11 June 2018: pp. 1-10.
- Kleeman, S.N., R.D. Adlard and R.J.G. Lester. 2002a. Detection of the initial infective stages of the protozoan parasite Marteilia sydneyi in Saccostrea glomerata and their development through to sporogenesis. International Journal for Parasitology 32: 767-784.
- Kleeman, S.N., F. Le Roux, F. Berthe and R.D. Adlard. 2002b. Specificity of PCR and in situ hybridization assays designed for detection of Marteilia sydneyi and M. refringens. Parasitology 125: 131-141.
- Le Roux, F., C. Audemard, A. Barnaud and F. Berthe. 1999. DNA Probes as potential tools for the detection of Marteilia refringens. Marine Biotechnology 1: 588-597.
- Le Roux, F., G. Lorenzo, P. Peyret, C. Audemard, A. Figueras, C. Vivarès, M. Gouy and F. Berthe. 2001. Molecular evidence for the existence of two species of Marteilia in Europe. The Journal of Eukaryotic Microbiology 48: 449-454.
- Longshaw, M., S.W. Feist, R.A. Matthews and A. Figueras. 2001. Ultrastructural characterisation of Marteilia species (Paramyxea) from Ostrea edulis, Mytilus edulis and Mytilus galloprovincialis in Europe. Diseases of Aquatic Organisms 44: 137-142.
- López-Flores, I., R. de la Herrán, M.A. Garrido-Ramos, J.I. Navas and M. Ruiz Rejón. 2004. The molecular diagnosis of Marteilia refringens and differentiation between Marteilia strains infecting oysters and mussels based on the rDNA IGS sequence. Parasitology 129: 411-419.
- López-Flores, I., M.A. Garrido-Ramos, R. de la Herran, C. Ruiz-Rejón, M. Ruiz-Rejón and J.I. Navas. 2008a. Identification of Marteilia refringens infecting the razor clam Solen marginatus by PCR and in situ hybridization. Molecular and Cellular Probes 22: 151-155.
- López-Flores, I., F. Robles, J.M. Valencia, A. Grau, A. Villalba, R. de la Herrán, M.A. Garrido-Ramos, C. Ruiz-Rejón, M. Ruiz-Rejón and J.I. Navas. 2008b. Detection of Marteilia refringens using nested PCR and in situ hybridisation in Chamelea gallina from the Balearic Islands (Spain). Diseases of Aquatic Organisms 82: 79-87.
- Mialhe, E., E. Bachère, C. LeBec and H. Grizel. 1985. Isolement et purification de Marteilia (Protozoa: Ascetospora) parasites de bivalves marins. (Isolation and purification of Marteilia (Protozoa: Ascetospora) parasites of marine Bivalvia: ultrastructural study of pansporoblasts.). Compte Rendu Hebdomadaire des Séances de l'Académie des Sciences, Paris. Série III 301: 137-142. (In French, with English summary).
- Novoa, B., D. Posada and A. Figueras. 2005. Polymorphisms in the sequences of Marteilia internal transcribed spacer region of the ribosomal RNA genes (ITS-1) in Spain: genetic types are not related with bivalve hosts. Journal of Fish Diseases 28: 331-338.
- OIE. 2012. Manual of Diagnostic Tests for Aquatic Animals 2017. Chapter 2.4.4. — Infection with Marteilia refringens. There is an OIE (World Organisation for Animal Health) Reference Laboratory for Infection with Marteilia refringens (consult the OIE Web site for the most up-to-date list.
- Pascual, S., A. Villalba, E. Abollo, M. Garci, A.F. González, M. Nombela, D. Posada and A. Guerra. 2010. The mussel Xenostrobus securis: a well-established alien invader in the Ria de Vigo (Spain, NE Atlantic). Biological Invasions 12: 2091-2103.
- Pëllumb, A., G. Ceschia and S. Kapllan. 2006. First report of Marteiliosis in Mytilus galloprovincialis in Albania. Ittiopatologia 3: 47-52.
- Pérez Camacho, A., A. Villalba, R. Beiras and U. Labarta. 1997. Absorption efficiency and condition of cultured mussels (Mytilus edulis galloprovincialis Linnaeus) of Galicia (NW Spain) infected by parasites Marteilia refringens Grizel et al. and Mytilicola intestinalis Steuer. Journal of Shellfish Research 16: 77-82.
- Perkins, F.O. 1976. Ultrastructure of sporulation in the European flat oyster pathogen, Marteilia refringens - taxonomic implications. Journal of Protozoology 23: 64-74.
- Pernas, M., B. Novoa, C. Tafalla and A. Figueras. 2000. Efficiency of different monoclonal antibodies in immunological assays developed for the detection of Marteilia sp. isolated from Mytilus galloprovincialis. Bulletin of the European Association of Fish Pathologists 20: 193-198.
- Pernas, M., B. Novoa, F. Berthe, C. Tafalla and A. Figueras. 2001. Molecular methods for the diagnosis of Marteilia refringens. Bulletin of the European Association of Fish Pathologists 21: 200-208.
- Rayyan, A., P. Damianidis, C. Antoniadou and C.C. Chintiroglou. 2006. Protozoan parasites in cultured mussels Mytilus galloprovincialis in the Thermaikos Gulf (north Aegean Sea, Greece). Diseases of Aquatic Organisms 70: 251-254.
- Robledo, J.A.F. and A. Figueras. 1995. The effect of culture-site, depth, season, and stock source on the prevalence of Marteilia refringens in cultured mussels (Mytilus galloprovincialis Lmk.) from Galicia, Spain. The Journal of Parasitology 81: 354-363.
- Robledo, J.A.F., V. Boulo, E. Mialhe, B. Desprès and A. Figueras. 1994a. Monoclonal antibodies against sporangia and spores of Marteilia sp. (Protozoa: Ascetospora). Diseases of Aquatic Organisms 18: 211-216.
- Robledo, J.A.F., J. Cáceres-Martínez and A. Figueras. 1994b. Marteilia refringens in mussel (Mytilus galloprovincialis Lmk.) beds in Spain. Bulletin of the European Association of Fish Pathologists 14: 61-63.
- Robledo, J.A.F., M.M. Santarém, P. González and A. Figueras. 1995a. Seasonal variations in the biochemical composition of the serum of Mytilus galloprovincialis Lmk. and its relationship to the reproductive cycle and parasitic load. Aquaculture 133: 311-322.
- Robledo, J.A.F., E. Mialhe and A. Figueras. 1995b. Purification of several phases of the parasite Marteilia (Protozoa: Ascetospora) from mussels (Mytilus edulis). In: Stolen, J.S., T.C. Fletcher, S.A. Smith, J.T. Zelikoff, S.L. Kaattari, R.S. Anderson, K. Söderhäll, B.A. Weeks-Perkins (eds.). Techniques in Fish Immunology, Immunology and Pathology of Aquatic Invertebrates, Vol. 4 Fish Immunology Technical Communications. SOS Publications, Fair Haven. pp. 117-121.
- Tige, G. and M.A. Rabouin. 1976. Étude d'un lot de moules transférées dans un centre touché par l'épizootie affectant l'huître plate. International Council for Exploration of the Sea C.M.1976/K:21: 10 pp. (In French with English abstract).
- Tiscar, P.G., M. Tempesta and M. Compagnucci. 1993. Peroxidase conjugated polyclonal antibody against Marteilia sp. purified from infected mussels (Mytilus galloprovincialis, Lmk) cultivated in Apulia, southern Italy. Bulletin of the European Association of Fish Pathologists 13: 53-55.
- Villalba, A., S.G. Mourelle, M.C. López, M.J. Carballal and C. Azevedo. 1993a. Marteiliasis affecting cultured mussels Mytilus galloprovincialis of Galicia (NW Spain). I. Etiology, phases of the infection, and temporal and spatial variability in prevalence. Diseases of Aquatic Organisms 16: 61-72.
- Villalba, A., S.G. Mourelle, M.J. Carballal and M.C. López. 1993b. Effects of infection by the protistan parasite Marteilia refringens on the reproduction of cultured mussels Mytilus galloprovincialis in Galicia (NW Spain). Diseases of Aquatic Organisms 17: 205-213.
- Villalba, A., S.G. Mourelle, M.J. Carballal and C. López. 1997. Symbionts and diseases of farmed mussels Mytilus galloprovincialis throughout the culture process in the Rías of Galicia (NW Spain). Diseases of Aquatic Organisms 31: 127-139.
- Virvilis, C., P. Angelidis and G. Photis. 2003. Presence of the parasite Marteilia sp. in the shellfish of the Thermaikos Gulf in northern Greece. Bulletin of the European Association of Fish Pathologists 23: 157-162.
- Wang, Z., X. Lu, Y. Liang and Z. Zheng. 2012. A Marteilia-like parasite in blue mussels Mytilus edulis in China. Journal of Aquatic Animal Health 24: 161-164.
- Zrnčić, S., F. Le Roux, D. Oraić, B. Šoštarić and F.C.J. Berthe. 2001. First record of Marteillia sp. in mussels Mytilus galloprovincialis in Croatia. Diseases of Aquatic Organisms 44: 143-148.
Citation information
Bower, S.M. (2019): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Marteilia spp. of Mussels.
Date last revised: November 2020
Comments to Susan Bower
- Date modified: