Mytilicola orientalis (Red Worm) of Clams and Cockles
On this page
Category
Category 2 (In Canada and of Regional Concern)
Common, generally accepted names of the organism or disease agent
Mytilicola disease, Red worm.
Scientific name or taxonomic affiliation
Mytilicola orientalis (Copepoda, family Mytilicolidae) [not a worm] (Mori 1935). In 1938, it was erroneously redescribed as Mytilicola ostreae (Lauckner 1983).
Geographic distribution
Mytilicola orientalis was originally described from oysters (Crassostrea gigas) and mussels (Mytilus crassitesta) in Japan (Mori 1935). This parasite is believed to have been introduced from Japan to the west coast of the United States with seed oysters as early as the 1930s and is now widely spread along the west coast of North America (including Canada's west coast). It was also introduced into France in the 1970s with imported C. gigas (Lauckner 1983, Grizel 1985). Following the implementation of EC Council Directive 91/67/EEC, the free movement of trade in shellfish commenced in January 1993 resulting in the range extension of M. orientalis from France to Ireland (Minchin et al. 1993, Minchin 1996). It was also recorded for the first time from the North Sea area (Netherlands) in 1993 (Stock 1993) and now also occurs in the Mediterranean Sea (Streftaris and Zenetos 2006).
Local (regional) distribution at least throughout the Northeastern Pacific, and probably elsewhere, seems restricted to sheltered muddy estuaries, where bivalves near the low tide mark seem to be most heavily infested. Goater and Weber (1996) attributed this distribution to factors that restrict colonization by the free-swimming larvae suggesting that wave action, tidal currents, salinity and/or substratum conditions may play a role.
Host species
Venerupis (=Tapes) philippinarum, Protothaca (=Venerupis) staminea, Nuttallia obscurata, Saxidomus giganteus, Crepidula fornicata and a wide range of other marine bivalves including oysters and mussels. In British Columbia, Marshall et al. (2003) reported a considerably higher prevalence in the varnish clam (N. obscurata, 60% to 64% infested) than in native littleneck clams (P. staminea, 8% to 4%) and Manila clams (V. philippinarum, 0% to 4%) obtained from the same beaches.
Impact on the host
The pathological effects of M. orientalis are controversial. Recent publications reported minimal impact of M. orientalis on the various species of hosts on the west coast of Canada and in Europe. For example, Marshall et al. (2003) did not detect a significant impact on clams from the Strait of Georgia, British Columbia. However, the prevalence and intensity of infestation tended to be low. Nevertheless, M. orientalis is considered as a serious pest by some scientists (Holmes and Minchin, 1995; Streftaris and Zenetos, 2006). Mytilicola orientalis can alter the morphology of the epithelial lining of the gut. Like the related species Mytilicola intestinalis, M. orientalis attaches to the gut wall with the distal segments of the second antennae which has two spine-like setae and terminates in a curved claw and can cause metaplastic changes in the gut of its host (Lauckner 1983). Occasionally focal mucosa is completely eroded and an appendage of the copepod may penetrate the underlying connective tissue with concurrent haemocyte infiltration (Fig. 1). Beneath the areas of epithelial metaplasia, a fibrosis-like response may occur in the connective tissue suggesting an attempt by the host to protect underlying tissue by encapsulation of the parasite (Lauckner, 1983). When present in large numbers or when a single specimen is located in a small section of the intestinal tract, M. orientalis may cause distension of the gut wall (Fig. 2). However, pathology is believed to be negligible or minimal in most cases. In European waters, dual infections with M. intestinalis can occur and warrants cautious observation for potential synergy between the two species of Mytilicola resulting in disease consequences for bivalve hosts (Stock 1993, Torchin et al. 2002). Streftaris and Zenetos (2006) included M. orientalis in their list of 100 worst invasive species into the Mediterranean Sea.
The life cycle of Mytilicola orientalis is not known but is probably like that of M. intestinalis (Cheng 1967). In British Columbia, there was a single reproductive period from June to late August and larval stages were in the water column for a short period and did not travel far (Bernard, 1969). Marshall et al. (2003) reported this parasite from the lumen of the stomach and intestine, with one in the digestive gland duct, of clams.
Diagnostic techniques
Gross Observations
Tease open the stomach and intestine of fresh whole bivalves to reveal reddish coloured elongate copepods. To aid detection, the dissected intestinal tract can be compressed between two glass plates prior to microscopic examination. Because of the relatively elongate morphology and small limbs of this parasitic copepod, it looks like a worm to the unaided eye, thus the common name of red worm. It has five thoracic segments each with paired posterolateral triangular protuberances (processes) followed by a genital segment and then a narrower abdomen with incomplete segmentation. The female is about 6 to 12 mm in length, 1.3 mm in greatest width and can have paired elongate ovisacs (about 7 mm in length each containing about 200 eggs) attached to the genital segment and possibly extending beyond the posterior end of the abdomen. The male is smaller than the female with a total length of about 2 to 5 mm and greatest width of about 0.5 mm (Grizel, 1985). The head of M. orientalis carries a median red eye spot, the first pair of antennae has four segments and the second has two. The second antennae are modified as a pair of stout hooks that are used as anchors for resisting expulsion from the host. There is an overall reduction in the length and complexity of the appendages in comparison to free living copepods.
The three species of Mytilicola can be differentiated by external morphological characteristics. The adult female of M. orientalis is 10-12 mm long with a pair of slender processes extending from the posterior corners of the head, whereas the adult female of M. intestinalis is about 8 mm long with smoothly rounded posterior corners of the head. In the adults of both sexes, the second antenna has two segments in M. orientalis and three segments in M. intestinalis. The posterolateral thoracic protuberances are more prominent in M. orientalis, except for the first pair which is absent in male M. orientalis. Both M. orientalis and M. intestinalis can be differentiated from Mytilicola porrecta (an intestinal parasite of various commercial molluscs in southeastern USA) which is shorter (female about 5 mm long) and has four segments on the second antenna and no mandibles. The adult male of M. porrecta has reduced posterolateral thoracic protuberances that are almost indiscernible. The claw of the maxilliped is short, stout and strongly hooked in comparison to the elongated and not strongly hooked maxilliped claw of male M. orientalis and M. intestinalis.
Histology
Examine body cross sections for large copepods within the lumen of the gut. Copepods may attach by hooked appendages to the intestine wall. Focal tissue metaplasia may be present in the intestinal epithelium.
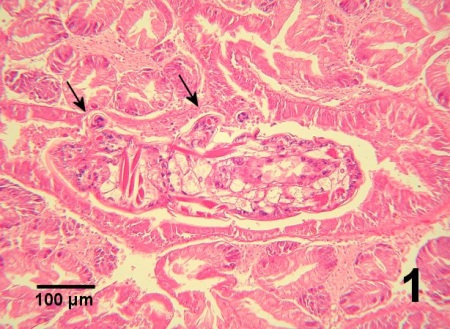
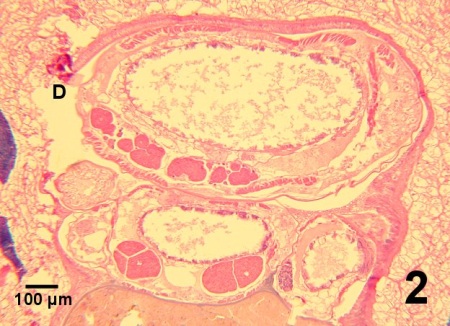
Digestion
Chemical disruption of tissues will expose copepods for easy quantification. Specifically, pepsin digestion of the flesh that was removed from the shells of bivalves followed by filtration of the disintegrated tissues through sieves (348 µm and 124 µm pore size) and examination of the residues for Mytilicola under a binocular microscope is a technique used for the detection of all parasitic stages M. intestinalis including egg sacs and early infective stages (0.45 µm long) intact (Dare, 1982). This process is recommended for large scale surveys rather than for diagnostic identity of the parasite.
Methods of control
No known methods of prevention or control. Bivalves from areas known to be affected (currently or historically) should not be moved to areas with no record of Mytilicola spp.
The risk of introduction lies in transplanting infested bivalves from one location to another. Currently, the greatest risk of introduction is associated with the bivalve aquaculture industry during transplantation and transportation of seed and farmed stocks. The reality of this risk has been exemplified by the introduction of M. orientalis from Japan to the west coast of North America in the 1930s and then to France in the 1970s and subsequently Ireland in 1993 through the transplanting of infested Crassostrea gigas (Steele and Mulcahy, 2001). The critical number of M. orientialis required to establish a population may depend on local conditions. Enclosed inlets with poor to moderate tidal flushing are more likely to develop local populations (Holmes and Minchin, 1995). The risk of introduction can be significantly reduced by the implementation of regulations that prohibit the movement of infested bivalves.
References
Bernard, F.R. 1969. The parasitic copepod Mytilicola orientalis in British Columbia. Journal Fisheries Research Board of Canada 26: 190-191.
Cheng, T.C. 1967. Marine molluscs as hosts for symbioses with a review of known parasites of commercially important species. In: F.S. Russell (ed.). Advances in Marine Biology. Volume 5. Academic Press Inc., London, p. 286-296.
Dare, P.J. 1982. The susceptibility of seed oysters of Ostrea edulis L. and Crassostrea gigas Thunberg to natural infestation by the copepod Mytilicola intestinalis Steuer. Aquaculture 26: 201-211.
Goater, C.P. and A.E. Weber. 1996. Factors affecting the distribution and abundance of Mytilicola orientalis (Copepoda) in the mussel, Mytilus trossulus, in Barkley Sound, B.C. Journal of Shellfish Research 15: 681-684.
Grizel, H. 1985. Mytilicola orientalis Mori, parasitism. In: C.J. Sindermann (ed.) Fiches d'Indentification des Maladies et Parasites des Poissons, Crustacés et Mollusques No. 20. ICES, Copengague. 4 pp.
Holmes, J.M.C. and D. Minchin. 1995. Two exotic copepods imported into Ireland with the Pacific oyster Crassostrea gigas (Thunberg). Irish Naturalists' Journal 25: 17-20.
Lauckner, G. 1983. Diseases of Mollusca: Bivalvia. In: O. Kinne (ed.). Diseases of Marine Animals. Volume II: Introduction, Bivalvia to Scaphopoda. Biologische Anstalt Helgoland, Hamburg, p. 829-830.
Marshall, W.L., S.M. Bower and G.R. Meyer. 2003. A comparison of the parasite and symbiont fauna of cohabiting native (Protothaca staminea) and introduced (Vennerupis philippinarum and Nuttalia obscurata) clams in British Columbia. Journal of Shellfish Research 22: 185-192.
Minchin, D. 1996. Management of the introduction and transfer of marine molluscs. Aquatic Conservation: Marine and Freshwater Ecosystems 6: 229-244.
Minchin, D., C.B. Duggan, J.M.C. Holmes and S. Neiland. 1993. Introductions of exotic species associated with Pacific oyster transfers from France to Ireland. International Council for Exploration of the Sea C.M. 1993/F: 27: 11 pp.
Mori, T. 1935. Mytilicola orientalis, a new species of parasitic Copepoda. Zoological Magazine, Tokyo 47: 687-690.
Steele, S. and M.F. Mulcahy. 2001. Impact of the copepod Mytilicola orientalis on the Pacific oyster Crassostrea gigas in Ireland. Diseases of Aquatic Organisms 47: 145-149.
Stock, J.H. 1993. Copepoda (Crustacea) associated with commercial and non-commercial Bivalvia in the East Scheldt, The Netherlands. Bijdragen tot de Dierkunde 63: 61-64.
Streftaris, N. and A. Zenetos. 2006. Alien marine species in the Mediterranean - the 100 ‘Worst Invasives’ and their impact. Mediterranean Marine Science 7: 87-118.
Torchin, M.E., K.D. Lafferty and A.M. Kuris. 2002. Parasites and marine invasions. Parasitology 124 Supplement: S137-S151.
Citation Information
Bower, S.M. (2010): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Mytilicola orientalis (Red Worm) of Clams and Cockles.
Date last revised: January 2010
Comments to Susan Bower
- Date modified: