Geoduck clam (Panopea generosa): Anatomy, Histology, Development, Pathology, Parasites and Symbionts
Dark Leathery Surface of Geoduck Clams
Gross appearance
Some geoduck clams collected from all areas sampled in British Columbia had dark thickened integument (periostracum) on the siphon and/or mantle that appeared brown or black (Figs. 1 to 4).




Figures 1 to 4. Pictures of fresh geoduck clams with dark thickened integument on the siphon and/or exposed muscular mantle.
A rust colour was observed on the mantle (Fig. 5) in some geoduck clams from each of the sites sampled.
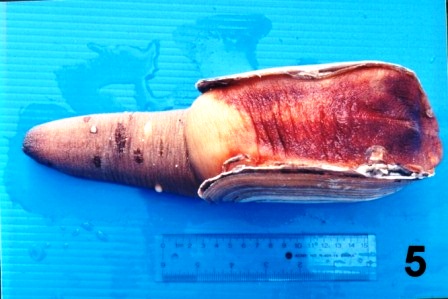
Figure 5. Ventral surface of a geoduck clam collected from the west coast of Vancouver Island (Fisheries Management Area 23) in February 1998 that had a rust tinted mantle.
Histopathology
In all instances, histological examination determined that the integument was affected in some manner but the underlying epithelium and musculature were always intact and healthy looking. A fungal infection was sometimes associated with the surface discolourations (thickened black/brown or rust coloured) of the siphon and/or the mantle of geoduck clams from the east and west coast of Vancouver Island (Fisheries Management Areas 14, 17, 23 and 24), but not in clams collected from two areas on the central coast of B.C. The fungus invaded the two acellular layers forming the integument of the siphon and mantle but did not penetrate into the epithelium nor into the musculature (Figs. 6 and 7). The fungal hyphae were septate and branching and produced thick walled, spherical fruiting bodies in the periostracum (Figs. 8 and 9).
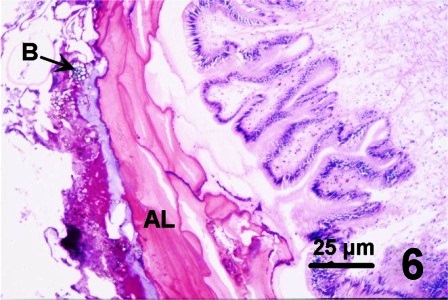
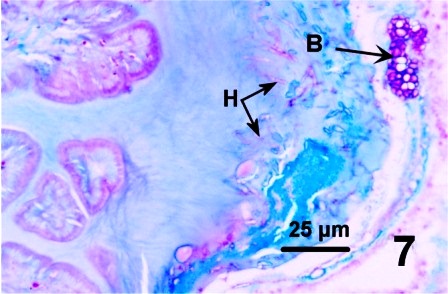
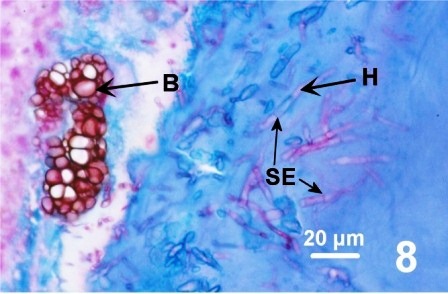
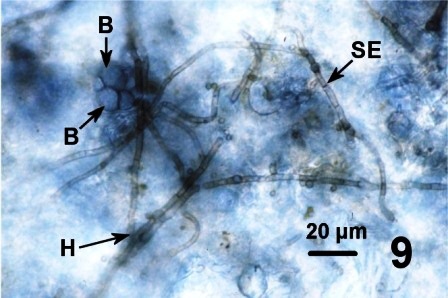
Figures 6 to 9. Histological sections (haematoxylin and eosin stain or PAS stain) and a wet mount preparation (unstained) of the fungus infecting the periostracum of geoduck clams.
Some clams with a thickened integument, but not infected with fungus (e.g., Fig. 1 and Fig. 2), possessed numerous layers of periostracum (both eosinophilic and lightly basophilic acellular layers) (Fig. 10). In other geoduck clams with areas of dark leathery integument, the outer acellular layer of the periostracum was inhabited by unknown organisms (Figs. 11 and 12). In a few cases, the periostracum was full of numerous holes, some of which were occupied by protozoa (Figs. 13 and 14). In other cases, both protozoa and the fungus were present (Fig. 15).
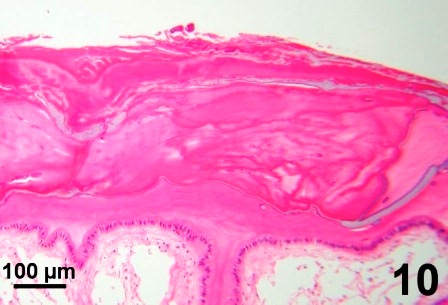


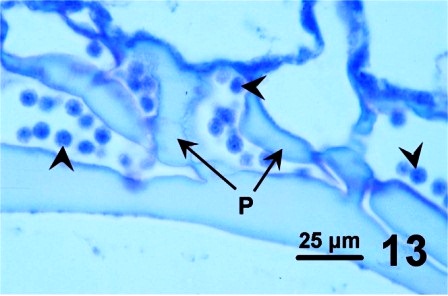
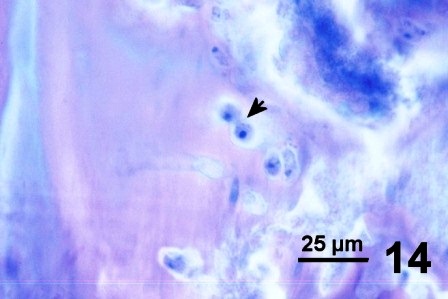
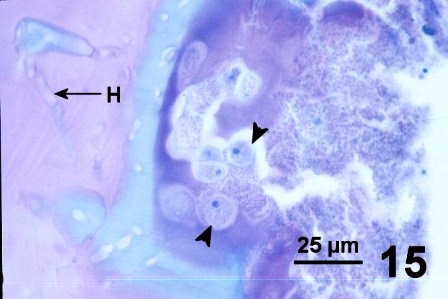
Figures 10 to 15. Various features and organisms associated with surface discolouration of the neck and mantle of geoduck clams. Haematoxylin and eosin stain.
In other geoduck clams with thickened brown or black integument or rust discolouration of the mantle but without associated fungus, various protozoa and multiple layers of periostracum, the periostracum was coated with a waxy looking acellular material that appeared yellow, black, and purple when stained with haematoxylin and eosin stain (Figs. 16 to 19). The origin of the yellow, black, and purple coating is unknown; it may be caused by local environmental conditions, be a reaction or development of the periostracum or be secreted by the geoduck clam. Material similar to the coating was observed in the kidney of the affected clams (Fig. 19). This may indicate that the clam kidney produces and secretes the material or is in the process of excreting material that was ingested.
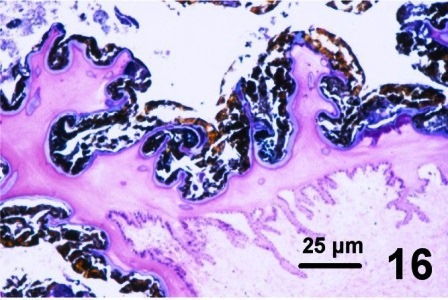
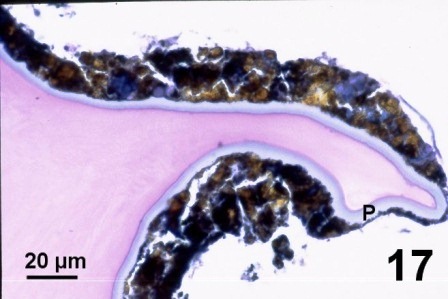
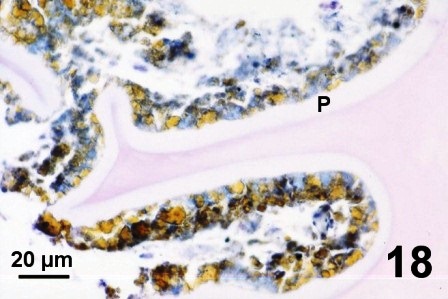
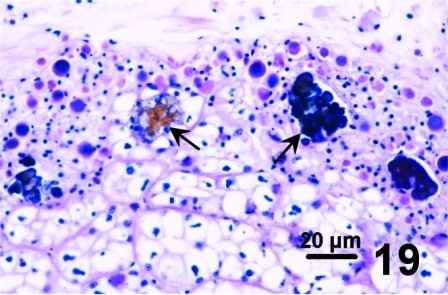
Figures 16 to 19. Histological appearance of the yellow, black and purple acellular material coating apparently normal periostracum and in the kidneys of geoduck clams with discoloured siphon and mantle. Haematoxylin and eosin stain.
Experimental attempt to transmit the fungus associated with periostracum discolouration
Two preliminary experiments were conducted in the laboratory to determine if the fungus encountered in some wild stocks of geoduck clams was infectious. In both experiments, healthy, unaffected cultured juvenile geoduck clams (year class 96) were utilised as potential fungal recipients. Cultured juvenile clams were obtained from grow-out sites on the east coast of Vancouver Island, maintained in the laboratory in ambient flow through seawater and fed approximately 4 litres of a combination of cultured algae [two flagellates (Isochrysis galbana and Pavlova lutheri) and a diatom (Thalassiosira pseudonana)] five times per week. In order to prevent the introduction of potential infectious disease agents from these clams into the local environment, all effluent was disinfected with chlorine prior to discharge to the Nanaimo city sewage system.
In the first experiment, transmission was attempted by contact with infected periostracum. On May 7, 1998, five juvenile geoduck clams (obtained after one year of grow-out near Texada Island) were placed in close proximity (in a 900 ml 'Scotty' bait jar in a tank with ambient flow through sea water) to strips of periostracum dissected from the siphon of an adult wild geoduck clam collected from the west coast of Vancouver Island (Fisheries Management Area 24) on May 5, 1998. The siphon of this clam was black and thickened, and a wet mount of the periostracum showed a heavy fungal infection (Fig. 9). After 82 days (on July 28) one juvenile was dead, and one had a black band around the siphon (Fig.20). Fungus was not detected in any of the histological preparations of these juvenile clams.
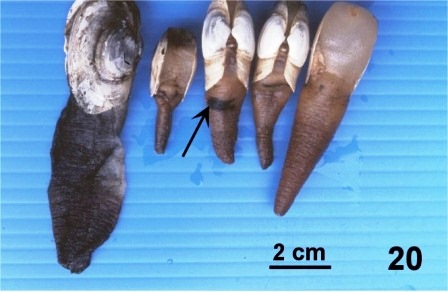
Figure 20. One dead juvenile (left side of picture) and four living juvenile geoduck clams that had been kept in close association with strips of fungus infected periostracum for 82 days. Note the black band (arrow) around the siphon of one clam.
In the second experiment, an attempt was made to transmit the fungus by prolonged contact with infected geoduck clams. Two sets of four juvenile geoduck clams (obtained after one year of grow-out near Jelina Island) were wrapped (with soft netting) against the siphons of two wild adult geoduck clams collected from the west coast of Vancouver Island (Fisheries Management Area 24) that were heavily infected with the fungus. After 85 days of contact (June 2 to August 26, 1998) none of the juvenile clams exhibited any gross indication of a fungal infection and fungus was not detected in any of the microscopic examination of the exposed juvenile clams.
In an effort to identify the periostracum fungus, an attempt was made to isolate the fungus using methods described for culturing the fungus Ostracoblabe implexa from the shells of oysters by Alderman and Jones (1971). On June 23, 1998, strips of fungus-infected periostracum dissected from an adult, wild geoduck clam collected from the west coast of Vancouver Island (Fisheries Management Area 24) on May 5, 1998 were placed in sterile, filtered sea water and stored at 4 °C. The sterile sea water was changed four times, over two days. On June 25th the washed fragments were aseptically transferred to sterile tubes containing sterile sea water with antibiotics (0.05 g penicillin G and 0.5 g streptomycin sulphate per 100 ml). One tube was incubated at room temperature and the other at 4 °C. The sterile sea water with antibiotics was changed daily over the first three days of incubation. After two weeks, mycelium growing from the edge of the periostracum and free swimming flagellates, possible fungal zoospores, were observed in the culture incubated at room temperature. To isolate the fungus, pieces of infected periostracum (about 3 mm by 4 mm in size) were aseptically removed, placed on agar plates (0.1 g yeast extract 0.1 g neopeptone [mycological], 0.7 g agar per 100 ml sea water), and incubated at room temperature, at 12.5 °C, or at 4 °C. Over 2 to 5 weeks vigorous growth of a fungus was observed at room temperature and lesser growth at 12.5 °C. The morphology of the fungal hyphae was similar (e.g. septate and branching), but the morphology of the fruiting bodies were very different from those observed on the geoduck clams. The fruiting bodies of the cultured fungus consisted of dark branchlets whereas the fruiting bodies of the fungus observed in the periostracum are thick walled spheres. Thus, these initial attempts were not successful in isolating the fungus that infects the periostracum of geoduck clams.
Results Summary: Attempts to transmit the fungus by prolonged contact and cohabitation were not successful after at least 82 days. Initial attempts to isolate the fungus on aseptic culture media also failed. Further research is required to determine the identity, distribution, and pathogenicity of the fungus associated with neck discolouration in geoduck clams from southern British Columbia.
Conclusion
Of 112 commercially harvested geoduck with abnormalities that were submitted for examination, 44 had dark thickened integument on the siphon, 25 had dark thickened integument on the mantle, and/or 58 had rust discolouration on the mantle. The apparent absence of fungus in the periostracum of some geoduck clams with black or brown thickened periostracum and/or rust tint of the mantle may be attributed to the relatively small tissue samples that were examined histologically. More sensitive methods of detecting the fungus and specific identification of the fungus are required before its involvement with the black and brown thickened condition can be fully assessed. Nevertheless, the association of various organisms (including fungus) with integument abnormalities suggests that the organisms may be secondary invaders taking advantage of altered periostracum for colonisation. If this premise is true, then the question of what caused the periostracum to be altered and made suitable for colonisation by various organisms must be addressed before the risk associated with the discolourations that affect market prices but not the health of the geoduck clams can be fully assessed.
Cáceres-Martínez et al. (2015) also reported a darkening and thickening appearance on the mantle and siphon of some P. generosa from the Pacific coast of Baja California, Mexico. In their study, all geoduck clams affected by darkening of the siphon showed cavernous layers in the surface of the periostracum and numerous protozoa were associated with the holes. Images of the protozoa presented by Cáceres-Martínez et al. (2015) are similar to those in Fig. 13 above. However, in British Columbia, the sponge-like holes and associated protozoa were observed in only a few of the geoduck clams with dark patches on the mantle and/or siphon. In addition, fungal hyphae were detected by Cáceres-Martínez et al. (2015) and in British Columbia (see Figs. 8 and 9 above). In both British Columbia and Mexico, further research is required to specifically identify the protozoa and fungi associated with the geoduck clam periostracum.
References
Alderman, D.J. and E.G.B. Jones. 1971. Shell disease of oysters. Fishery Investigation, London (Series 2) 26(8): 1-19.
Cáceres-Martínez, J., R. Vásquez-Yeomans and R. Cruz-Flores. 2015. First Description of symbionts, parasites, and diseases of the Pacific geoduck Panopea generosa from the Pacific coast of Baja California, Mexico. Journal of Shellfish Research 34: 751-756.
Fisheries Management Area locations as mentioned in above text can be viewed on the map.
Citation Information
Bower, S.M. and Blackbourn, J. (2003): Geoduck clam (Panopea generosa): Anatomy, Histology, Development, Pathology, Parasites and Symbionts: Dark Leathery Surface of Geoduck Clams.
Date last revised: August 2020
Comments to Susan Bower
- Date modified: