Perkinsus of Clams and Cockles
On this page
Category
Category 1 (Not Reported in Canada)
Common, generally accepted names of the organism or disease agent
Clam Perkinsus disease, Perkinsosis of Clams. The specific identity of some of these parasites has not yet been fully confirmed. Reports pertaining to the same parasite were assigned a letter code which is consistently applied to all available information on that parasite under each of the following headings.
Scientific name or taxonomic affiliation
The interpretation of the taxonomic affiliation of the genus Perkinsus has changed over time as indicated in the disease description for Perkinsus marinus. Taxonomic investigations into the affiliation with the relatively new phylum Perkinsozoa were conducted using Perkinsus olseni (=atlanticus) (Teles-Grilo et al. 2007a, b) and Perkinsus chesapeaki (Zhang et al. 2011). Zhang et al. (2011) supported the affiliation of the genus Perkinsus with an independent lineage (Perkinsozoa) positioned between the phyla of Apicomplexa and Dinoflagellata. Following is information on the specific names applied to species of Perkinsus reported in clams.
- Perkinsus olseni (=atlanticus). This parasite was initially described as Perkinsus atlanticus from clams in Europe (Azevedo 1989). Subsequently, a species of Perkinsus was found in Venerupis (=Tapes, =Ruditapes) philippinarum in Korea, China and Japan. Hamaguchi et al. (1998) found the nucleotide sequence of two internal transcribed spacers (ITS1 and ITS2) and the 5.8 S region of the RNA to be almost identical to P. atlanticus and Perkinsus olseni and suggested that the parasite in Japan may be P. atlanticus. Perkinsus olseni was originally described from abalone but cross-infection experiments and molecular studies suggested that only one species of Perkinsus occurs in a wide variety of molluscs, including clams, in Australia (Goggin et al. 1989, Goggin and Lester 1995). Further molecular studies identified considerable similarities between P. atlanticus and P. olseni (Berth 2004). Note: Because the synonymy of P. olseni and P. atlanticus proposed by Murrell et al. (2002) is upheld, the name P. olseni has priority. Using the same regions of the DNA and sequences of a non-transcribed spacer (NTS), Park et al. (2005, 2008) determined that the Perkinsus sp. from Korea was homologous to the species from Japan and identified it as Perkinsus olseni (=atlanticus). Elandaloussi et al. (2009a, b) also found isolates from Spain homologous to P. olseni and determined that despite the broad geographic range of this parasite, P. olseni strains group together according to their host rather than their geographic origins within a well-resolved P. olseni clade. Thus, P. olseni has a wide host range (infecting gastropods as well as bivalves) and a wide geographic range (including at least the coasts of Australia, New Zealand, Vietnam, Japan, Korea, China, Europe and Uruguay).
- Perkinsus chesapeaki from Mya arenaria, identity based on minor differences in the morphology of the zoospore and molecular differences from Perkinsus marinus (McLaughlin et al. 2000b). At least two species of Perkinsus (P. marinus and P. chesapeaki) occur in M. arenaria (Kotob et al. 1999a, b; McLaughlin et al. 2000a). Crassostrea virginica, Macoma balthica and M. arenaria were experimentally susceptible to infection by mantle cavity inoculations of P. chesapeaki cultured isolates (Dungan et al. 2007a). Although P. chesapeaki was experimentally infective for oyster and clam hosts, a survey of wild bivalves in the Chesapeake Bay region revealed that P. chesapeaki infections predominated among members of at least 6 clam species and rarely (6%) occurred in C. virginica (Reece et al. 2008).
- Perkinsus (=Labyrinthomyxa) andrewsi from Macoma balthica was differentiated from Perkinsus marinus, Perkinsus atlanticus, Perkinsus olseni and Perkinsus qugwadi based on sequence data from the rRNA locus (Coss et al. 2001b). DNA analysis (using polymerase chain reaction (PCR) assays based on regions of the ribosomal RNA SSU loci (mainly ITS1 and ITS2) indicated that this species can occur in other clams (Macoma mitchelli and Mercenaria mercenaria) as well as oysters (Crassostrea virginica) where it can coexist with Perkinsus marinus (Coss et al. 1999, 2001b). However, P. andrewsiwas relatively rare (1.3%) in comparison to P. marinus detected in 58.4% of the 394 C. virginica examined during an investigation (Pecher et al. 2008). Analysis of the ITS regions of several species of Perkinsus (including several isolates of some species) consistently grouped P. chesapeaki and P. andrewsi (Murrell et al. 2002, Casas et al. 2002a, b). Also, analysis of the ITS sequence from cloned isolates of Perkinsus sp. from Mya arenaria and Tagelus plebeius from Chesapeake Bay suggested that the variations among ITS sequences of P. chesapeaki and P. andrewsi indicates true polymorphism within a single parasite species (Dungan et al. 2002). This variability was confirmed by Pecher et al. (2004) who reported a second rRNA gene unit in P. andrewsi in which all regions (except for the 5.8S) exhibited sequence differences from that initially described for this parasite and this second rRNA gene was similar to variations reported by Dungan et al. (2002). Note: If P. chesapeaki and P. andrewsi are synonymous, the name P. chesapeaki will have precedence over P. andrewsi (Burreson et al. 2003). A detailed study by Burreson et al. (2005) which included morphological (in vivo and in vitro), molecular (sequence analysis of three genetic loci: ribosomal RNA (rRNA) internal transcribed spacer (ITS) regions, rRNA large subunit (LSU) gene and actin gene) and experimental (reciprocal cross-infection) research, supported the synonymy of the two species. However, Pecher et al. (2008) considered this evidence to be too limited to support the synonymy. Specifically, Pecher et al. (2008) argued that P. chesapeaki was original described as a distinct morphotype by McLaughlin et al. (2000b) and the Perkinsus isolate that was analyzed to clarify the relationship of P. andrewsi and P. chesapeaki was designated as P. chesapeaki because it was isolated from the appropriate type host. Because this isolate appears to be morphologically identical to P. andrewsi, it may not be the P. chesapeaki that was originally described. Thus, the P. andrewsi designation was retained by Percher et al. (2008) who await additional evidence to support the synonymy despite the claim by Burreson et al. (2005) that the neohapantotype culture of P. chesapeaki was morphologically identical to the published morphology for P. chesapeaki and was obtained from the type host and from the same general area of Chesapeake Bay as the type locality.
- Perkinsus sp. with a high level of sequence homology in the ITS-5.8S rRNA region to that of P. olseni and P. atlanticus (99.85% and 99.71%, respectively) and less homology (94.88% or greater) to that of other species (i.e., Perkinsus marinus, Perkinsus andrewsi and Perkinsus qugwadi). Like wise, the NTS rRNA region was only slightly different from that of P. olseni (1.31%) and P. atlanticus (3.73%) and highly different from that of P. marinus (24.62%) and P. andrewsi (53.45%). The species was not assigned in this case because the level of homology required to discriminate between species of Perkinsus had not been determined (Leethochavalit et al. 2003).
- Perkinsus honshuensis with distinct differences in morphological and genetic characteristics from those of P. olseni (Dungan and Reece 2006).
- Perkinsus mediterraneus originally described from oysters (Casas et al. 2004) is phylogenetically closely related, but a distinct sister clade to the Perkinsus olseni (=atlanticus) group that occured in clams from the same area (Cao et al. 2008).
Note 1: A Perkinsus atlanticus-like protist isolated in vitro from the clam Ruditapes decussatus in Galicia, Spain had a small subunit (SSU) ribosomal RNA gene sequence unlike that published for P. olseni (= atlanticus) in GenBank. This parasite was tentatively named "Pseudoperkinsus tapetis" and affiliated with fungus-like protists in the recently named Mesomycetozoa (Figueras et al. 1992, 1996, 2000). Like Perkinsus spp., this isolate developed large prezoosporangia (hypnospores) that stained dark blue with Lugol's iodine stain after incubation in FTM (Figueras et al. 2001). Thus, the Ray's FTM assay cannot be used to differentiate between the two species (Novoa et al. 2002). Also, the protease activity of Pseudoperkinsus tapetis extracellular products was different from those described for Perkinsus marinus (Ordás et al. 2001b).
Note 2: Perkinsus spp. cannot be discriminated on the basis of morphology, host species or geographic location. Even with molecular data, there can be problems recognizing true species boundaries if multiple clonal cultures are not established, if too few DNA clones are sequenced and if cultures are from only a restricted host or geographic range (Burreson et al. 2005). Recommendations for describing new species of Perkinsus were presented by Burreson et al. (2005).
Geographic distribution
- France (Atlantic and Mediterranean coasts (Miossec et al. 2006) including at least four different French marine areas; Morbihan gulf, Arcachon bay, Leucate and Thau lagoons (Arzul et al. 2009) ), Portugal, Spain (including Galicia and Delta de l'Ebre, Catalonia (NW Spain), Huelva coast (SW Spain), Andalucia (S. Spain), Balearic Islands and Mediterranean coast), and Italy (the Mediterranean Sea and the NW Adriatic Sea); Great Barrier Reef, South Australia, northern Western Australia, northern New Zealand and in an Vietnamese ornamental clams (Tridacna crocea) imported into the U.S.A. (Sheppard and Phillips 2008, Sheppard and Dungan 2009); west and south coasts of South Korea, in Kumamoto, Hiroshima and Mie Prefectures of Japan, and Bohai Sea, along the northern coast of the Yellow Sea and the southern coast of China; and the coast of Uruguay. Perkinsus olseni (=atlanticus) was also tentatively identified from Macoma balthica in a tributary of Chesapeake Bay, USA (Kleinschuster et al. 1994).
- Chesapeake Bay (McLaughlin and Faisal 2000) and Delaware Bay (Bushek et al. 2008), USA. Unidentified Perkinsus species were detected by Ray's FTM and genus-specific PCR (as described by Robledo et al. 2002) in Mercenaria mercenaria from the Gulf of Mexico coast of Florida (McCoy et al. 2007). Perkinsus chesapeaki was detected in Venerupis (=Ruditapes) philippinarum from one location in Galicia, Spain (Ramilo et al. 2012) and in the same species of clam from Bonne Anse in Charente Maritime, on the Atlantic coast of France and in Ruditapes decussatus from Leucate, a lagoon on the Mediterranean coast of France (Arzul et al. 2012).
- Virginia and Maryland (Chesapeake Bay) to Maine, USA (Pecher et al. 2008). Unidentified Perkinsus species were detected by Ray's FTM and genus-specific PCR (as described by Robledo et al. 2002) in Mercenaria mercenaria from the Gulf of Mexico coast of Florida (McCoy et al. 2007).
- Gulf of Thailand in Chonburi Province, Thailand.
- Gokasho Bay, Mie Prefecture, Japan.
- Menorca (Balearic Islands) Spain.
Host species
- Ruditapes (=Tapes, =Venerupis) decussatus, Ruditapes (=Tapes) semidecussatus, Tapes (=Venerupis) rhomboides, Venerupis aurea, Venerupis (=Ruditapes) pullastra, Venus verrucosa and imported/cultured Venerupis (=Tapes, =Ruditapes) philippinarum introduced into France in the mid 1970's (Flassch and Leborgne 1992). In France, epizootiological surveys in 2004 and 2005 found prevalence and parasite burden higher in R. decussatus compared to V. philippinarum with no associated mortality (Arzul et al. 2009). This parasite was also detected in all samples examined during a two year survey of R. decussatus from five locations along the coast of Portugal and one location in Galicia, Spain (Leite et al. 2004). In Galicia, Spain, P. olseni was also detected in Venerupis senegalensis and Tapes rhomboides (Ramilo et al. 2012). In the North-Western Adriatic Sea (Italy), Perkinsus sp. (probably P. olseni) has also been detected in Cerastoderma edule, Chamelea gallina, Callista chione and other bivalves (Da Ros and Canzonier 1985, Canestri-Trotti et al. 2000) and in the area of Sardinia from Cerastoderma glaucum (Culurgioni et al. 2006). Many species of molluscs including Tridacna gigas, Tridacna maxima, Tridacna crocea, Anadara trapezia, Hippopus hippopus, Chama iostoma, Chama pacificus, Acrostengma unicolor and Katelysia rhytiphora from the southwestern Pacific Ocean including Macomona liliana, Barbatia novaezealandiae, and Austrovenus stutchburyi from northern New Zealand were infected (Goggin and Lester 1987, Hine 2002, Hine and Diggles 2002, Murrell et al. 2002, Dungan et al. 2007b). In northern Western Australia, a survey detected Perkinsus sp. in a wide diversity of bivalve molluscs including species of clams such as Barbatia helblingii (Hine and Thorne 2000). Venerupis (=Tapes, =Ruditapes) philippinarum and Protothaca jedoensis but not observed in 10 other molluscs (including Crassostrea gigas and Pinctada fucata martensii) from enzootic areas in South Korea, Japan and China (Choi and Park 1997; Park et al. 2001; Park et al. 2006a, 2008; Wu et al. 2011). However, DNA of P. olseniwas detected in Crassostrea ariakensis in its native range (China, Japan and Korea) and in Crassostrea hongkongensis from the south coast of China via molecular diagnostic screening using PCR based assays (Moss and Reece 2005, Moss et al. 2007). Also found in the commercial clam Pitar rostata from Uruguay (Cremonte et al. 2005). Crassostrea virginica and Mercenaria mercenaria were found susceptible to P. olseni via experimental exposure (inoculations and bath challenges with cultured or directly harvested parasites) in the laboratory (Moss et al. 2008).
- Originally described from Mya arenaria which is designated as the type host. Also occurs in Macoma balthica, Tagelus plebeius, Macoma mitchelli, Mercenaria mercenaria, Mulinia lateralis, Rangia cuneata, Cyrtopleura costata, and Crassostrea virginica on the east coast of the U.S.A. (Burreson et al. 2005, Reece et al. 2008) and in Venerupis philippinarum and Ruditapes decussatus in France and Spain (Arzul et al. 2012, Ramilo et al. 2012). Arzul et al. (2012) used in situ hybridization tests to confirm the presence of both P. chesapeaki and P. olseni in the same R. decussatus population and even in the same clams.
- Macoma balthica designated as type host but also occurs in Macoma mitchelli, Mercenaria mercenaria and Crassostrea virginica (Coss et al. 2001b, Pecher et al. 2008).
- Paphai undulata (Leethochavalit et al. 2003, 2004).
- Venerupis philippinarum. Note that at least two Perkinsus spp. (P. olseni and P. honshuensis) infect this species of Japanese clam (Dungan and Reece 2006, Park et al. 2008).
- Venus verrucosa from the Mediterranean coast of Spain and sometimes in mixed infections with P. olseni (Cao et al. 2008).
Impact on the host
In most clam species, the parasite frequently induces the formation of visible milky white cysts or nodule on the gills, foot, gut, digestive gland, kidney, gonad and mantle of heavily infected clams. The sometimes massive aggregation of Perkinsus sp. and haemocytes form lesions that may interfere with respiration and other physiological processes such as reproduction (fertility/fecundity, when large lesions occur in the gonads), growth and/or survival and thus have an impact on fishery/aquaculture productivity. However, as indicated in the following paragraphs, the impact of Perkinsus spp. on clams seems to be highly varable and may depend on various conditions including the species/variety of Perkinsus, the identity of the host and environmental factors.
On the coasts of Europe, infection in Ruditapes decussatus has been associated with extensive mortalities in clam breeding areas located on the south coast of Portugal (Azevedo et al. 1990). However, on the Galician coast of Spain, perkinsosis did not appear to affect the energetic physiology of infected R. decussatus at about 15 °C but, Villalba and Casas (2001) speculated that higher temperatures may impact on disease severity. Villalba et al. (2005) detected an annual pattern of infection with lowest mean intensity and prevalence of infection occurring during the winter and a peak of infection occurring in the spring that was significantly associated with seawater temperature at about 15 °C Also, clams from a perkinsosis-affected area had a significantly higher mortality than clams from a non-affected area (e.g., 7.0% and 2.8%, respectively), in early September, the time of year with the highest mortality (Villalba et al. 2005). However, Leite et al. (2004) detected no significant differences in the intensity of Perkinsus sp. infections in R. decussatus between samples collected in winter and summer from Portugal although, major differences were observed from year to year and site to site indicating that factors other than those responsible for seasonal climatic variations might affect the prevalence and intensity of infection for this parasite. Similar results were reported by Elandaloussi et al. (2008) who observed no obvious seasonality in the prevalence of P. olseni in V. philippinarum and R. decussatus from the NW Mediterranean Sea and there was no significant correlation between the intensity of infection in these clams and either seawater temperature or salinity. Also, Dang et al. (2010) detected no seasonal cycle of P. olseni in V. philippinarum at 34 stations throughout Arcachon Bay (SW France) where the prevalence of infection was high (on average between 70 and 100%). A two-year survey for P. olseni in V. philippinarum and R. decussatus along the coasts of France revealed a lower prevalence of infection along the Atlantic coast and English Channel than in the Mediterranean Sea but no abnormal mortalities were detected (Miossec 2006, Miossec et al. 2006). Montes et al. (2001) determined that P. olseni (=atlanticus) parasitism favours the development of opportunistic infections by bacteria and viruses which have detrimental effects in clam (R. semidecussatus) populations from the northern Mediterranean coast of Spain. In Galicia, Spain, P. olseni inhibited the gonadal development of R. decussatus and the haemocytic response to the parasite reduced the volume of the gonadal tissue. However, no significant effect of infection on gonadal index, fecundity or spawning efficiency was observed (Casas and Villalba 2012). Alternately, results of field studies by Dang et al. (2013) indicated that concentrations of between 105 and 106 cells of Perkinsus (likely P. olseni but possibly mixed with P. chesapeaki at one site) per gram of gill tissue, affected the physiological functions of V. philippinarum and R. decussatus in France and Spain, respectively, highlighted by impact on the growth rate of the clams.
On the eastern coast of Asia, Perkinsus sp. was considered as the cause of epizootic mass mortalities and decline in commercial harvest for the decade prior to 2005 of Venerupis philippinarum in Korea (Park et al. 1999, Choi and Park 2005) and China (Liang et al. 2001, Wu et al. 2011) and the cause of populations declines of this clam in Japan (Hamaguchi et al. 1998, Park et al. 2008). Also, heavy infections observed in older clams in Korea appeared to cause retarded growth and delayed gamete maturation resulting in altered population dynamics and stability (Park and Choi 2001). Specifically, high level of Perkinsus infection affects spawning frequency and reduces egg production in V. philippinarum, which may have long-term impacts on clam recruitment and population growth (Park et al. 2006b). Also, in Korea, results of surveys for Perkinsus sp. in V. philippinarum indicated that the spatial distribution of this parasite is in some way controlled by temperature, salinity and substrate type (Park and Choi 2001). Nago and Choi (2004) found that the prevalence and intensity of Perkinsus was lowest in September and highest in March in V. philippinarum from Jeju, an island off the south coast of Korea. However, Choi and Park (2005) and Yang et al. (2012) reported that infection intensity was highest in the fall (September to November) when the physiological condition of clams was poor after spawning and mass mortalities were observed in the clam beds on the west and south coast of South Korea. In five locations on the coast of east China, the prevalence of P. olseni in V. philippinarum ranged from 43.75 to 95.83%, and was significantly higher in October than in May (Wu et al. 2011). In laboratory studies, P. olseni caused direct mortality in V. philippinarum juveniles (3 to 10 mm shell length) and the lethal level of infection was estimated at approximately 107 pathogen cells per gram of soft tissue (Shimokawa et al. 2010). However, results of clam disease surveys in Korea by Lee et al. (2001) indicated that caution should be applied when determining a causal relationship between Perkinsus sp. infections and V. philippinarum mortalities. In Japan, during laboratory and field studies, Yoshinaga et al. (2010) did not obtain clear evidence showing the negative impact of P. olseni on the physiology (clearance of diatoms (Cheatoceros calcitrans) from sea water, tolerance of high water temperatures and burrowing activity) and survival of infected V. philippinarum. Also, a significant decrease in the intensity of infection was noted in November at the end of the spawning period (Yoshinaga et al. 2010).
Gills appear to be the main target tissues for most Perkinsus spp. in clams. In Mya arenaria, the most commonly observed lesions were clusters of trophozoites encapsulated in well-circumscribed walls forming a cyst-like structure. In advanced infections, the plethora of cysts in the branchial connective tissue was accompanied by loss of underlying tissue structures and gill lamellae (McLaughlin and Faisal 1998a). Similar observation were made by Park and Choi (2001), Lee et al. (2001) and Choi and Park (2005) for Perkinsus sp. in V. philippinarum from Korea. Leite et al. (2004) found a significant decline in the condition index (percentage between the edible part and the total weight of the clams) of R. decussatus that were heavily infected with Perkinsus. Goggin (1996) reported that P. olseni did not cause tridacnid clams (specifically, Tridacna crocea), up to 100 mm shell length, to lose wet tissue weight.
Haemocytes, especially granulocytes, of R. decussatus in vitro were able to phagocytose trophozoites but not zoospores of P. olseni (=atlanticus) (López et al. 1997). In addition, secretion products from cultures of P. olseni (=atlanticus), which contained high protein concentration, acid phosphatase and protease activity, inhibited the phagocytosis of various particles, specifically zymosan, Escherichia coli and Vibrio tapetis by R. decussatus and mussels Mytilus galloprovincialis (Ordás et al. 1999). McLaughlin et al. (2000a) and McLaughlin and Faisal (2001) reported a difference in the production of extracellular proteins by P. chesapeaki and P. marinus that may help to explain the difference in pathology observed in infected Mya arenaria and Crassostrea virginica, respectively. Hégaret et al. (2007) found that V. philippinarum infected with P. olseni maintain haemocyte function but their immune system response to harmful or toxic algal exposure was modified by parasite infection. In addition, da Silva et al. (2008) determined that the prevalence and intensity of P. olseni decreased in clams exposed to the same toxic algae (Karenia selliformis). Also, P. olseni affected the pathological status of V. philippinarum exposed to cultures of the toxic algae Prorocentrum minimum, by causing atrophy and degeneration of residual ova in the gonadal follicles and hyaline degeneration of the muscle fibers, indicating synergistic effects of both stressors on the host over a short period of time (Hégaret et al. 2009). In vitro, cells and/or exudates of K. selliformis and P. minimum were detrimental to P. olseni by causing altered morphology and increased percentage of dead P. olseni (da Silva et al. 2008, Hégaret et al. 2009). Overall, initial exposure of P. olseni-infected clams to some toxic algae appeared to modify the host–parasite interaction by causing effects in both the host and its parasite thus, suggesting antagonistic suppression of transmission and proliferation of the parasite in the natural environment by some harmful algal blooms over a longer period of time (da Silva et al. 2008, Hégaret et al. 2009). Possibly, some harmful algal blooms may mitigate rather than exacerbate parasite effects (Carnegie 2011). However, such effects are dependant on the identiy of the toxic algae. For example, Hégaret et al. (2012) reported that the toxic dinoflagellate Alexandrium ostenfeldii did not cause a significant decrease in P. olseni intensity within clams and did not affect infected clam burrowing capacity, nor condition index, nor digestive enzyme activities. However, exposure of infected clams to A. ostenfeldii had a significant impact on histopathology (increase in circulating haemocytes, desquamation and haemocyte diapedesis in the intestinal tract and gills) and increased the production of reactive oxygen species in haemocytes (Hégaret et al. 2012).
Ruditapes decussatus and R. semidecussatus developed a defensive response to P. olseni (=atlanticus) which included differentiation of recruited granular haemocytes in the vicinity of the parasite and the de novo secretion of the polypeptide p225 (Montes et al. 1996, Montes et al. 1997). Ordás et al. (2000) determined that advanced infections in R. decussatus had a measurable effect on defence parameters, especially anti-bacterial activity and agglutination titres (lectins) in the haemolymph. Kim et al. (2006) and Kang et al. (2006, 2008) also detected lectin production in V. philippinarum infected with P. olseni in South Korea. However, in contrast, da Silva et al. (2008) found a low impact of P. olseni on the immune system of V. philippinarum, from Brittany, France, with no induction of lectin production. Immunofluorescence staining (using polyclonal monospecific IgG antiserum produced in rabbits) revealed that the lectin from V. philippinarum bound to the surfaces of purified (hypnospores) of Perkinsus sp. from Korea (Bulgakov et al. 2004). Examination of the molecular basis of host-pathogen interactions in R. decussatus against P. olseni resulted in the detection of differentially expressed gene sequences in the haemocytes and gills of infected clams (Prado-Alvarez et al. 2009).
Two iron chelators, desferrioxamine and 2,2'-bipyridyl, with differential binding properties were able to inhibit P. olseni (=atlanticus) proliferation in vitro in a dose-dependent manner but this effect was cytostatic and reversed by removal of the chelators or co-addition of iron indicating a high degree of susceptibility of P. olseni to chelator-induced iron deprivation (Elandalloussi et al. 2003). When tested in vivo in R. decussatus only desferrioxamine was found to be effective in reducing infections of P. olseni and this chelator had no observed acute toxicity (mortality) in Perkinsus-free clams (Elandalloussi et al. 2005a).
After reviewing epidemiological methods currently used in field studies to evaluate the occurrence of P. olseni in clams, Miossec et al. (2005) proposed recommendations for a basic methodological design to conduct an epidemiological survey. These recommendations included: clearly defining the target population, identifying sampling methodology (preferably as probabilistic as possible), calculate sample size according to survey objectives, evaluate the quantitative values of sensitivity and specificity for the diagnostic test used, and apply additional tools to fully characterize (identify) the pathogen (Miossec et al. 2005).
Diagnostic techniques
Gross Observations
Infected clams may have whitish nodules or cysts on the surface of the gill, digestive gland and mantle tissues due to a haemocytic response by the clam to Perkinsus spp.
Wet Mounts
Spherical bodies containing an eccentric vacuole (signet-ring) in cysts from moribund clams.
Histology
Systemic proliferation of haemocytes in response to immature trophozoites (=aplanospores), mature trophozoites (="signet-ring" or aplanospore with large eccentric vacuole that displaces the nucleus to the periphery of the cell), and tomonts (="rosette", sporangium, schizont or palintomic cells) stages of parasite development. In comparison to Perkinsus marinus from Crassostrea virginica, P. olseni (=atlanticus) from clams in Europe can have larger trophozoites (30-40 µm in diameter) but generally diameter varies from 3 to 15 µm and the trophozoites of Perkinsus sp. from V. philippinarum in Japan vary in size from 2 to 32.5 µm with average diameters of 12 to 15 µm. Arzul et al. (2012) found no consistent size differences between cells of P. olseni (9·1 ± 2·8 μm, n=161) and P. chesapeaki (9·8 ± 2·9 μm, n=58) from clams in France. In most clams, infection is usually associated with an infiltration of numerous haemocytes into the surrounding tissues (Ordás et al. 2001a). Typical lesions were haemocyte-encapsulated granulomatous cysts in haemocyte-infiltrated connective tissues, containing Perkinsus cells that were often enrobed in an amorphous eosinophilic matrix apparently secreted by the clam haemocytes (Arzul et al. 2012). Occasionally, the Perkinsus cells occur within granular haemocytes and Sheppard and Phillips (2008) reported a radiating corona pattern in the eosinophilic material surrounding some trophozoites. Lesions usually occur in gill and digestive system connective tissues and less frequently in gonad, mantle, kidney, and heart connective tissues (Dungan and Reece 2006).
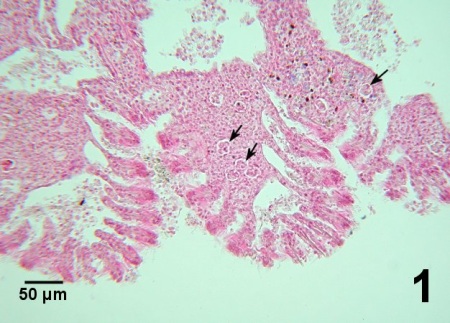
Figure 1. Extensive infiltration of haemocytes around clusters of Perkinsus sp. (arrows) causing congestion of the gills in Venerupis (=Ruditapes) philippinarum from Korea. Haematoxylin and eosin stain.
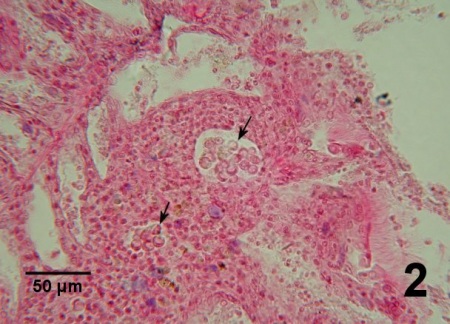
Figure 2. Two clusters of many Perkinsus sp. (arrows) within an accumulation of haemocytes in the connective tissue of the gill of V. philippinarum from Korea. Haematoxylin and eosin stain.

Figure 3. Two clusters of mature trophozoites (arrows) surrounded by haemocytes in the gills of V. philippinarum from Korea. Haematoxylin and eosin stain. Images in Figs. 1-3 obtained from histological section kindly provided by Dr. K.-S. (Albert) Choi, Cheju National University, South Korea.
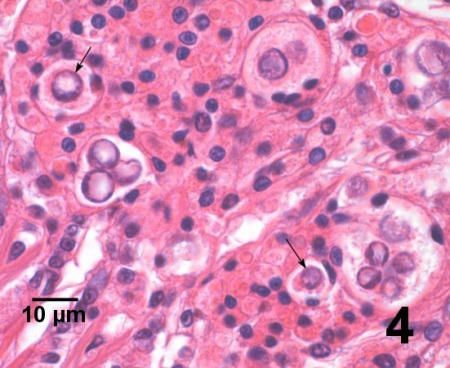
Figure 4. Another example of several clusters and single trophozoites (arrows) surrounded by haemocytes in the gills of V. philippinarum from Korea. Haematoxylin and eosin stain.
In lightly infected M. arenaria, Perkinsus sp. usually occur in the gill lamellae, either free or surrounded (often encapsulated) by haemocytes, which appears to result in fusion between adjacent lamellae. Often clusters of trophozoites embedded in amorphous eosinophilic material and tissue debris formed cysts (17.8 ± 7.9 µm, range of 8 to 44 µm) in the gills. Entrapped trophozoites were circular or oval (3.8 ± 1.4 µm in diameter), uninucleate and each contained a large vacuole that occupied most of the cell. In more heavily infected M. arenaria, the cysts increased in number and in size (47.6 ± 12.8 µm, range of 24 to 68 µm) and their amorphous outer wall became more demarcated from the surrounding tissue. In advanced infections, Perkinsus sp. cells predominated the internal structure of the gill lamellae and subepithelial connective tissue. Cysts were also observed in the connective tissue between the tubules of the digestive gland, in the gonads and kidneys and were often associated with large lesions consisting of free and encapsulated Perkinsus sp. cells and haemocytes within an eosinophilic matrix. Perkinsus sp. propagated by schizogony with tomonts (7.9 ± 1.6 µm in diameter, range of 6 to 12 µm) containing up to 4 daughter cells (McLaughlin and Faisal 1998). Burreson et al. (2005) reported that host reactions to P. chesapeaki infections varied considerably in the three clam species (M. arenaria, T. plebeius and M. balthica) that have been studied histologically and this resulted in some differences in parasite sizes and morphologies observed in these different hosts.
Histopathological examination of clams often detect fewer infected clams than Ray's FTM assay (Rodríguez and Navas 1995, Almeida et al. 1999, Leethochavalit et al. 2004, Dungan et al. 2007b). To date, no well-defined morphological features have been identified for differentiating between the various species of Perkinsus reported from clams and other molluscs. Also, trophozoite morphology does not have taxonomic value because it can be influenced by the host, the time of the year, and nutrient availability (Villalba et al. 2004).
Electron Microscopy
Chagot et al. (1986) and Comps and Chagot (1987) provided a brief description and a few ultrastructural images of a Perkinsus in R. decussatus from Portugal and Sagristà et al. (1995, 1996) provided observations of the cellular response of the host and a detailed account of zoosporulation of a Perkinsus in V. philippinarum from the Mediterranean coast of Spain. Also, the fine structure of clonally propagated in vitro life stages of Perkinsus andrewsi were described (Coss et al. 2001a). However, all features observed were consistent with those of other species of Perkinsus described from various molluscs.
Immunological Assay
An antiserum prepared against Perkinsus marinus (polyclonal antibodies, prepared by C.F. Dungan, Cooperative Oxford Laboratory, Oxford, MD, USA) cross reacted will trophozoites of Perkinsus sp. in histological sections of V. philippinarum from Japan (Maeno et al. 1999). Montes et al. (2002) used immunological techniques to localize a main proteinaceous component of the cell wall of P. olseni (=atlanticus) and determined that polyclonal antibodies to the protein cross-reacted with Perkinsus marinus. Rabbit anti-P. olseni IgG prepared by Park et al. (2010) was specific to all life stages, including the prezoosporangium, trophozoite, and zoospore by an immunofluorescent assay and was used to isolate P. olseni prezoosporangium-like cells from marine sediment collected from the west coast of Korea where P. olseni–associated clam mortality had recurred for the past decade. To date, no diagnostic assay based on anti-Perkinsus sp. antibodies has been rigorously validated, and antibodies that have been produced may exhibit cross-reactivity with dinoflagellates (Villalba et al. 2004).
DNA Probes
The internal transcribed spacers (ITS region including ITS1 and ITS2 and the connecting 5.8S region), the small subunit (SSU or 18S), the large subunit (LSU) and/or the non-transcribed spacer (NTS) of the ribosomal RNA locus and/or the actin gene of some Perkinsus isolates from various clams (and other bivalves) have been sequenced. Sequences have been compared between various isolates from various molluscs and species synonymy was proposed based on sequence similarities. For example, only slight differences (0.8%) were found between the ITS region of Perkinsus from clams and cockles and Perkinsus olseni from abalone in Australia (Goggin 1994). Hamaguchi et al. (1998) found this sequence in Perkinsus sp. from V. philippinarum in Japan to be almost identical (99.9%) to that reported by Goggin and Barker (1993) and Goggin (1994) from P. atlanticus, P. olseni and other Perkinsus sp. isolates. Casas et al. (2002a, b) also reported that the ITS sequence from 13 isolates of Perkinsus sp. from Ruditapes decussatus in Galicia (NW Spain) were closely matched with equivalent sequences from P. atlanticus, P. olseni, and Perkinsus sp. from Chama pacificus and A. trapezia. This synonymy was supported by Park et al. (2005) for Perkinsus from V. philippinarum in Korea and by Elandaloussi et al. (2009a, b) for Perkinsus from clams (V. philippinarum and R. decussatus) in Spanish Mediterranean waters. In these publications, the gene sequences of the P. olseni (=atlanticus) isolates were distinct from those of P. marinus and also different from a cluster consisting of P. chesapeaki and P. andrewsi. Thus, the synonymy of P. olseni and P. atlanticus proposed by Murrell et al. (2002) is well supported by molecular evidence.
Molecular sequences have also been used to identify new species. For example, the nucleotide sequences (ribosomal DNA internal transcribed spacer region, the large subunit rRNA gene, and actin genes) of one of four Perkinsus isolates from V. philippinarum in Gokasho Bay, Japan, that was morphologically unique, differed from those of all described Perkinsus species and was named Perkinsus honshuensis (similar sequences from the other three isolates were consistent with those reported for P. olseni) (Dungan and Reece 2006). However, because Perknisus spp. tend not to be host specific and are morphologically similar, species identification based on rRNA sequence differences has resulted in controversy between investigators. For example, P. chesapeaki and P. andrewi initially described from different bivalves from Chesapeak Bay are now reported to occur in a similar range of host bivalves. Also, isolates from these host species are now known to have multiple polymorphic sequences in the rRNA gene with sequence similarities occurring in isolates identified as P. chesapeaki and P. andrewsi (Dungan et al. 2002, Percher et al. 2004). To further complicate the issue, corresponding type material was not deposited for each species (i.e., P. chesapeaki is represented by a type histological slide but no holotype in vitro culture isolate is available while P. andrewsi is represented by a holotype clonal culture but no type histological slide was deposited) making it difficult to compare the two species from original material. In an attempt to address the problem, Burreson et al. (2005) demonstrated that both parasites were indistinguishable based on molecular, morphological and experimental evidence and supported the synonymy of the two species. However, Pecher et al. (2008) retained the P. andrewsi designation because the neohapantotype culture isolate of P. chesapeaki presented by Burreson et al. (2005) appeared to be morphologically identical to P. andrewsi. Thus, Pecher et al. (2008) stated that the neohapantotype culture isolate may not be the P. chesapeaki originally described and await additional evidence to support the synonymy.
Various polymerase chain reaction (PCR) based diagnostic assays proposed to be genus-specific and/or species-specific have been developed (Reece et al. 2001, Robledo et al. 2002, Casas et al. 2002a, Park et al. 2005). For example: Hamaguchi et al. (1998) designed a PCR method for the diagnosis of the Perkinsus sp. from V. philippinarum in Japan; Elston et al. (2003) used in situ hybridization to verify that the parasite in V. philippinarum from Korea was Perkinsus sp.; Kotob et al. (1999a, b) used sequence analysis of the ITS regions of two isolates of Perkinsus sp. from Mya arenaria to suggest that the two isolates were different species of Perkinsus and Balseiro et al. (2010) identified a nested PCR assay for P. olseni to improve on the PCR assay described by Kotob et al. (1999b); Robledoet al. (1999), Cross et al. (2001b) and Percher et al. (2008) developed PCR-based diagnostic assays for P. andrewsi in the USA; Moss et al. (2006) and Reece et al. (2008) designed P. olseni and P. chesapeake species-specific primers, respectively, for PCR and in-situ hybridization (ISH) assays that were used by Arzul et al. (2012) to detect both parasites in clams from France; Robledo et al. (2000) and De la Herrán et al. (2000) developed a PCR-based diagnostic assay for P. olseni (=atlanticus) from Spain that was used by Costa et al. (2012) to detect the parasite in southern Portugal; Elandalloussi et al. (2004) developed a PCR-enzyme-linked immunosorbent assay (ELISA) for amplification of an IGS sequence region and rapid detection of Perkinsus species in which the specific hybridisation of DIG-labelled amplified products to species-specific capture probes was detected colourimetrically; and Abollo et al. (2006) developed a species-specific PCR fragment length polymorphism (PCR-RFLP) assay of the rRNA ITS region that used a single restriction enzyme (Rsa I) to discriminate P. chesapeaki and P. marinus and a combination of two endonucleases (Rsa I plus Hinf I) to discriminate P. olseni and P. mediterraneus. Arzul et al. (2009, 2012) employed the PCR procedures described by Casas et al. (2002a) as well as PCR procedures that targeted other segments of the genome (large subunit rRNA genes and actin-1 genes) and the RFLP of Abollo et al. (2006) followed by sequencing of products to identify the species of Perkinsus detected in clams from France. However, as cautioned by Burreson (2000), more research is necessary to compare PCR assays with standard diagnostic techniques before PCR can be recommended as the method of choice for perkinsosis diagnosis. For example, primers that target the NTS, a region with high inter-specific variation, have demonstrated good species specificity (Coss et al. 2001b, Park et al. 2008). However, intra-specific variations within the NTS region has not been broadly assessed creating a risk of false negatives due to polymorphism within a species if the PCR primers do not bind the target sequence of all strains of that species (Villalba et al. 2004). Also, further research is required to resolve which DNA sequence differences are consistently significant in the identification of species and how these differences relate to biological parameters that can be used to describe and differentiate between closely related species. This is especially important if some species and/or strains of Perkinsus prove to be non-pathogenic for some or all host species. Nevertheless, molecular sequence data is playing an increasingly important role in the identification of Perkinsus species and requires adequate DNA sequence data at the targeted loci from the same and related species over a wide geographic area in order to develop reliable, accurate and sensitive molecular diagnostic tools (Villalba et al. 2004).
A loop-mediated isothermal amplification (LAMP) assay was designed to target the conserved internal transcribed spacer 2 (ITS2) region of the SSU rRNA gene of Perkinsus spp. (Feng et al. 2013). Although this LAMP assay was apparently validated using clam samples collected from coastal areas in eastern China known to be infected with Perkinsus olseni, Feng et al. (2013) also claimed that it detected P. marinus in oysters imported from Australia where the parasite is not known to occur according to the World Health Organisation for Animal Health Manual of Diagnostic Tests for Aquatic Animals 2013 and Australia's National List of Reportable Diseases of Aquatic Animals 2011. Other components of the genome in Perkinsus spp. in clams have been described but to date, none of these have been developed into diagnostic assays (Ascenso et al. 2009, Pardo et al. 2011, Zhang et al. 2011, Marques et al. 2012).
Culture
Examine tissues which have been placed in Fluid Thioglycollate Medium (FTM) for approximately 7 days for Lugol's iodine positive prezoosporangia (hypnospores), up to 250 µm in diameter (Ray's FTM, see Ray (1966) for details of this technique and Nickens et al. (2002) for an alternative formulation). Although not true propagating cultures, this procedure is used for the diagnosis of many species of Perkinsus but may also detect other organisms (Villalba et al. 2004) and thus cannot be used to distinguish Perkinsus to species (Elandaloussi et al. 2008). The usual diameter of Perkinsus sp. prezoosporangia from FTM were reported as 30 to 40 µm from R. decussatus from Portugal (Azevado 1989) and 25 to 75 µm from P. undulata from Thailand (Leethochavalit et al. 2004). False negative diagnosis have occurred in 46%, 22% and 13% of infected clams (R. decussatus and V. philippinarum in Spain) in which only haemolymph, gills and the remaining body, respectively, were assayed by Ray's FTM (=thioglycollate diagnosis, Rodriguez and Navas 1995). However, Villalba et al. (2005) claimed that the examination of two gill lamellae from R. decussatus processed by Ray's FTM was more sensitive, quicker and cheaper than examination of histological sections. Although the examination of the whole-clam soft tissues by Ray's FTM allowed for the detection of very light infections, the correlation between the infection intensity estimated by both assays (whole-clam soft tissues and two gill lamellae) was high (Villalba et al. 2005). For Perkinsus sp. in M. arenaria, Ray's FTM of gill and palp tissue is more sensitive than assaying either rectal tissue or haemolymph in light infections and more sensitive than histological examination. But, the use of both rectal and gill tissues in Ray's FTM was recommended (McLaughlin and Faisal 1999). Bushek et al. (2008) indicated that for P. chesapeaki, the difference in detection capabilities between Ray's FTM and PCR based detection assays related to the quality and type of tissues processed rather than assay sensitivity per se and further cautioned to use care when applying and interpreting diagnostic assays used on novel species.
Moore et al. (2002) determined that trophozoites of P. olseni from A. trapezia made nonviable with formalin, irradiation or colchicine failed to swell in FTM and did not differentially stain in Lugol's iodine. However, trophozoites that had already developed into prezoosporangia in FTM and subsequently rendered inactive by freezing, ethanol or formalin retained their iodinophilic properties and could be used as a partial control for the Ray's FTM test. Choi and Park (1997) described a method of determining the number of Perkinsus sp. in V. philippinarum by digesting clam tissues from FTM in sodium hydroxide (2M NaOH), followed by washing (via centrifugation) and counting a subsample, stained with Lugol's iodine, using a haemocytometer. Park et al. (2006b) used this method on gill tissue from V. philippinarum to estimate the intensity of infection in each clam. Almeida et al. (1999) also determined that lysis in 2M NaOH at 60 °C for 1 to 3 hours after incubating an entire minced or homogenized clams in FTM for 4 days followed by centrifugation to remove the NaOH supernatant and staining with Lugol's iodine solution was a quantitative diagnostic procedure. This procedure was more sensitive than histology for detecting low levels of infection (Almeida et al. 1999). Slight modifications of this procedure (FTM incubation followed by 2M NaOH digestion and then staining with Lugol's iodine) was used by other researchers to determine the body burden or intensity of infection of Perkinsus in clams (Leite et al. 2004, Yoshinaga et al. 2010, Yang et al. 2012, Dang et al. 2013).
Optimal conditions for zoosporulation of prezoosporangia was in samples washed free of FTM and incubated in sea water at 24-28 °C, 25-35 ppt salinity, and pH 7-8. Ahn and Kim (2001) determined that temperature and salinity had significant effects on zoosporulation of Perkinsus sp. in V. philippinarum in Korea. Prezoosporangia isolated from clams during the winter sporulated and released motile zoospores at 10 °C and 5 parts per thousand salinity but, prezoosporangia isolated during the summer did not sporulate at 10 °C and low salinities (10 parts per thousand or less) had a significant negative impact on development. Virus-like particles were observed in trophozoite-like cells isolated from gill tissues of R. decussatus that had been incubated in FTM (Azevedo 1990).
Perkinsus olseni (= atlanticus) in the haemolymph from the adductor muscle of R. decussatus was propagated in vitro and found to adapt to very different culture media, salinity (tolerance for 15 to 40 ppt) and temperature conditions (within extremes of 5 and 37 °C and optimums of 16 to 26 °C), and the inoculum density did not affect final cell concentrations attained (Ordás and Figueras 1998). Robledo et al. (2002) developed an in vitro clonal culture of this parasite. The culture media described by Robledo et al. (2002) was used to investigate the influence of specific drugs on the proliferation and metabolic pathways of P. olseni (Elandalloussi et al. 2005a, b). Perkinsus sp. in haemolymph from M. M. could also be propagated in nutrient medium as for P. marinus from Crassostrea virginica (Coss et al. 2001a, Arzul et al. 2009). La Peyer et al. (2006) identified another nutrient medium that they used to determine the effects of salinity on viability, metabolic activity and proliferation of P. marinus, P. olseni and P. chesapeaki. La Peyre et al. (2008) determined that in vitro, P. olseni declined in metabolic activity and proliferation from 28 °C to 15 °C and was viable after 30 days incubation at 4 °C, but had limited metabolic activity and no proliferation. Casas et al. (2002b) reported a low frequency of zoosporulation (<1% of dividing cells) in continuous cultures of P. olseni (=atlanticus) isolated from Ruditapes (=Tapes) decussatus. Also, their cultured cells enlarged in FTM and stained blue-black with Lugol's iodine which is characteristic for this parasite from infected clams. Alternatively, Burreson et al. (2005) obtained in vitro isolates of Perkinsus sp. from prezoosporangia produced in FTM and reported that P. chesapeaki proliferated in cultures by both schizogony (multiple internal divisions in trophozoites enlarged to about 15 µm in diameter to produce clusters of sibling daughter trophozoites that subsumed the mother cell biomass, ~75% of dividing cells) and zoosporulation (a series of reductive divisions to yield hundreds of motile zoospores within a zoosporangium that were between 25 to 85 µm in diameter, ~25% of dividing cells). Zoospores released into the culture medium were motile for about 24 hours, shed their flagella, enlarged into typical trophozoites (about 9 µm in diameter with vacuolated, "signet-ring" morphology including an eccentric nucleus bearing a prominent nucleolus) and underwent subsequent schizogony or zoosporulation (Burreson et al. 2005). Dungan and Reece (2006) also used the same procedure and culture medium as Burreson et al. (2005) to obtain isolates of Perkinsus spp. from V. philippinarum from Japan. Differences in size were reported by Arzul et al. (2012) for in vitro stages of P. olseni and P. chesapeake grown under identical conditions with the later species being larger than the former, especially for tomonts (=schizonts) and zoosporangia. Microsatellite markers identified in clonal cultures of P. olseni from laboratory isolates derived from infected R. decussatus (from Spain), V. philippinarum (from Spain and Japan), and Austrovenus stutchburyi (from New Zealand) show that (1) in vitro P. olseni are diploid cells, and (2) multiple infections can occur within a single host (Pardo et al. 2011). Cryopreserved isolates of Perkinsus spp. are available at the American Type Culture Collection (ATTC, Rockville, MD, USA, www.atcc.org).
Note: Balseiro et al. (2010) compared three diagnostic techniques to detect P. olseni in various species of clams from Galicia, Spain. They determined that nested PCR was appropriate for rapidly screening large numbers of clams. It showed high sensitivity and good correlation between research groups, was faster than histopathology and Ray's FTM and less expensive than histopathology. Also, nested PCR required less specialized training for technicians than histology. Although Ray's FTM lacked analytical specificity and gave divergent results between research groups, particularly in the case of low levels of infection, it was useful for disease-monitoring purposes.
Methods of control
No known methods of prevention in enzootic areas. Areas of the world where Perkinsus sp, is not known to occur (e.g., the Pacific coasts of north and central America) must be especially diligent in screening seed from enzootic regions prior to importation for grow-out (Elston et al. 2003). Park et al. (2010) found that the fecal discharge (feces and pseudofeces) and decomposition of infected clam tissue could be the two major P. olseni routes of transmission. In
France (Arcachon Bay), infection acquisition of P. olseni by V. philippinarum appeared to be episodic within spatially defined areas (Dang et al. 2010). In the management of perkinsosis, it must be kept in mind that at least for P. olseni (=atlanticus) in Spain, zoosporulation can occur in a wide range of temperatures (15 to 32 °C) and salinity (10 to 35 ppt) (with optimum values of 19 to 28 °C and 25 to 35 ppt), prezoosporangia survive up to 129 days at 10 °C, and zoospores survived for more than 20 days at various temperatures between 10 and 28 °C (Villalba et al. 2000). Goggin et al. (1990) determined that trophozoites of Perkinsus sp. in the tissues of blood cockles (Anadara trapezia) from Australia survived for at least 197 days at -60 °C. Cigarría et al. (1997) indicated that clam mortalities could be minimized by avoiding stressful conditions such as high densities, harvesting stress or overcrowding in depuration plants during the warmer months (water temperatures greater than 20 °C; apparently temperatures below 15 °C prevent Perkinsus sp. propagation in T. decussatus), as well as implementing two prophylactic measures of removing sets with parasitized clams and putting out (planting) unparasitised juveniles (seed) clams in aquaculture areas.
References
Abollo, E., S.M. Casas, G. Ceschia and A. Villalba. 2006. Differential diagnosis of Perkinsus species by polymerase chain reaction-restriction fragment length polymorphism assay. Molecular and Cellular Probes 20: 323-329.
Ahn, K.J. and K.H. Kim. 2001. Effect of temperature and salinity on in vitro zoosporulation of Perkinsus sp. in Manila clams Ruditapes philippinarum. Diseases of Aquatic Organisms 48: 43-46.
Almeida, M., F. Berthe, A. Thébault and M.T. Dinis. 1999. Whole clam culture as a quantitative diagnostic procedure of Perkinsus atlanticus (Apicomplexa, Perkinsea) in clams Ruditapes decussatus. Aquaculture 177: 325-332.
Arzul, I., J. Michel, B. Chollet, M. Robert, L. Miossec, C. Garcia and C. François. 2009. Molecular characterization of parasites of the genus Perkinsus present in clams from French producing areas. Journal of Shellfish Research 28(3): 681. (Abstract, for electronic version of presentation see https://archimer.ifremer.fr/doc/00000/6269/).
Arzul, I., B. Chollet, J. Michel, M. Robert, C. Garcia, J.-P. Joly, C. François and L. Miossec. 2012. One Perkinsus species may hide another: characterization of Perkinsus species present in clam production areas of France. Parasitology 139: 1757-1771.
Ascenso, R.M.T., R.B. Leite, R. Afonso and M.L. Cancela. 2009. Expression pattern of Perkinsus olseni genes in response to bivalves with different susceptibility to perkinsosis. Journal of Fish Diseases 32: 633-636.
Auzoux-Bordenave, S., A.M. Vigario, F. Ruano, I. Domart-Coulon and D. Doumenc. 1995.In vitro sporulation of the clam pathogen Perkinsus atlanticus (Apicomplexa, Perkinsea) under various environmental conditions. Journal of Shellfish Research 14: 469-475.
Azevedo, C. 1989. Fine structure of Perkinsus atlanticus n. sp. (Apicomplexa, Perkinsea) parasite of the clam Ruditapes decussatus from Portugal. The Journal of Parasitology 75: 627-635.
Azevedo, C. 1990. Virus-like particles in Perkinsus atlanticus (Apicomplexa, Perkinsidae). Diseases of Aquatic Organisms 9: 63-65.
Azevedo, C., L. Corral and R. Cachola. 1990a. Fine structure of zoosporulation in Perkinsus atlanticus (Apicomplexa: Perkinsea). Parasitology 100: 351-358.
Azevedo, C., L. Corral, R. Cachola and F.O. Perkins. 1990b. Fine structure of a new parasite (Perkinsus-like species) of Ruditapes decussatus (Bivalvia) from Portugal. In: F.O. Perkins and T.C. Cheng (eds.). Pathology in Marine Science. Academic Press, San Diego, p. 181-187.
Balseiro, P., J. Montes, R.F. Conchas, B. Novoa and A. Figueras. 2010. Comparison of diagnostic techniques to detect the clam pathogen Perkinsus olseni. Diseases of Aquatic Organisms 90: 143-151.
Berthe, F. 2004. Report about mollusc diseaes. Mediterranean aquaculture diagnostic laboratories 49: 33-48 (for electronic version see: https://archimer.ifremer.fr/doc/00000/3300/).
Bower, S., E. Burreson and K. Reece. 2003. Annex 10: Review of molecular techniques used to differentiate the various species/isolates of Perkinsus. Report of the Working Group on Pathology and Diseases of Marine Organisms, Aberdeen, UK, 11-15 March 2003. Mariculture Committee, ICES CM 2003/F:03, Ref. ACME, pg. 54-60 (for electronic version see: https://www.ices.dk/sites/pub/Publication%20Reports/Expert%20Group%20Report/mcc/2003/wgpdmo03.pdf (pg 60-66 of 101)).
Bulgakov, A.A., K.I. Park, K.S. Choi, H.K. Lim and M. Cho. 2004. Purification and charaterisation of a lectin isolated from Manila clam Ruditapes philippinarum in Korea. Fish and Shellfish Immunology 16: 487-499.
Burreson, E.M. 2000. Disease diagnosis by PCR: foolproof or fool hardy? Journal of Shellfish Research 19: 642. (Abstract).
Burreson, E.M., K.S. Reece, K.L. Hudson and C.F. Dungan. 2003. Perkinsus chesapeaki and Perkinsus andrewsi are the same species. Journal of Shellfish Research 22: 321. (Abstract).
Burreson, E.M., K. Reece and C.F. Dungan. 2005. Molecular, morphological, and experimental evidence support the synonymy of Perkinsus chesapeaki and Perkinsus andrewsi. Journal of Eukaryotic Microbiology 52: 258-270.
Bushek, D., B. Landau and E. Scarpa. 2008. Perkinsus chesapeaki in stout razor clams Tagelus plebeius from Delaware Bay. Diseases of Aquatic Organisms 78: 243-247.
Canestri-Trotti, G., E.M. Baccarani, F. Paesanti and E. Turolla. 2000. Monitoring of infections by protozoa of the genera Nematopsis, Perkinsus, and Porospora in the smooth venus clam Callista chione from the North-Western Adriatic Sea (Italy). Diseases of Aquatic Organisms 42: 157-161.
Cao, A., E. Abollo, B.G. Pardo, P. Martinez and A. Villalba. 2008. Identification of the Perkinsus spp. occurring in the Spanish coast and evaluation of their intraspecific variability. Journal of Shellfish Research 27: 994. (Abstract).
Carnegie, R.B. 2011. Contemporary issues in molluscan health: challenges and opportunities. In: OIE Global Conference on Aquatic Animal Health Programmes: Their Benefits for Global Food Security (Panama City, Panama), pp. 89-96. (for access to presentation see: http://www.oie.int/eng/A_aquatic/en_presentations.htm).
Casas, S.M. and A. Villalba. 2012. Study of perkinsosis in the grooved carpet shell clam Ruditapes decussatus in Galicia (NW Spain). III. The effects of Perkinsus olseni infection on clam reproduction. Aquaculture 356–357: 40-47.
Casas, S.M., A. Villalba and K.S. Reece. 2002a. Study of perkinsosis in the carpet shell clam Tapes decussatus in Galicia (NW Spain). I. Identification of the aetiological agent and in vitro modulation of zoosporulation by temperature and salinity. Diseases of Aquatic Organisms 50: 51-65.
Casas, S.M., J.F. La Peyre, K.S. Reece, C. Azevedo and A. Villalba. 2002b. Continuous in vitro culture of the carpet shell clam Tapes decussatus protozoan parasite Perkinsus atlanticus. Diseases of Aquatic Organisms 52: 217-231.
Casas, S.M., K.S. Reece, Y. Li, J.A. Moss, A. Villalba and J.F. La Peyer. 2008. Continuous culture of Perkinsus mediterraneus, a parasite of the European flat oyster Ostrea edulis, and characterization of its morphology, propagation, and extracellular proteins in vitro. Journal of Eukaryotic Microbiology 55: 34-43.
Chagot, D., M. Comps, V. Boulo, F. Ruano and H. Grizel. 1986. Etude histopathologique d'une reaction cellulaire chez Ruditapes decussatus infecte par un protozoaire. (Histological study of a cellular reaction in Ruditpaes decussatus infected by a protozoan). In: Azevedo, C. (ed.). Second International Colloquium on Pathology in Marine Aquaculture, Porto, Portugal, pp. 37-38. (original in French, for electronic English version see: https://archimer.ifremer.fr/doc/00000/3109/ and paper English version published in 1987 see Aquaculture 67: 260-261).
Choi, K.-S. and K.-I. Park. 1997. Report on the occurrence of Perkinsus sp. in the Manila clams, Ruditapes philippinarum in Korea. Journal of Aquaculture 10: 227-237.
Choi, K.-S. and K.-I. Park. 2005. Current status of Perkinsus infection in Korean waters. In: Walker, P.J., R.G. Lester, M.G. Bondad-Reantaso (eds.) Diseases in Asian Aquaculture V. Proceedings of the 5th Symposium on Diseases in Asian Aquaculture. Fish Health Section, Asian Fisheries Society, Manila. pp. 263-274. (for access to electronic version of symposium proceedings see: http://www.fhs-afs.net/publications.htm).
Choi, K.-S., K.-I. Park, K.-W. Lee and K. Matsuoka. 2002. Infection intensity, prevalence, and histopathology of Perkinsus sp. in the Manila clam, Ruditapes philippinarum, in Isahaya Bay, Japan. Journal of Shellfish Research 21: 119-125
Cigarría, J., C. Rodríguez and J.M. Fernández. 1997. Impact of Perkinsus sp. on Manila clam Ruditapes philippinarum beds. Diseases of Aquatic Organisms 29: 117-120.
Comps, M. and D. Chagot. 1987. Une parasitose nouvelle chez la palourde Ruditapes decussatus. Comptes Rendus Académie Sciences Paris, Série III 304: 41-44. (in French, for Open Access version see: https://archimer.ifremer.fr/doc/00000/3116/).
Coss, C.A., J.A.F. Robledo, G.R. Vasta and G.M. Ruiz. 1999. Identification of a new Perkinsus species isolated from Macoma balthica by characterization of the ribosomal RNA locus, evidence of its presence, simultaneous with P. marinus, in Crassostrea virginica, Macoma mitchelli and Mercenaria mercenaria. Journal of Shellfish Research 18: 318. (Abstract).
Coss, C.A., J.A.F. Robledo and G.R. Vasta. 2001a. Fine structure of clonally propagated in vitro life stages of a Perkinsus sp. isolated from the baltic clam Macoma balthica. The Journal of Eukaryotic Microbiology 48: 38-51.
Coss, C.A., J.A.F. Robledo, G.M. Ruiz and G.R. Vasta. 2001b. Description of Perkinsus andrewsi n.sp. isolated from the baltic clam (Macoma balthica) by characterization of the ribosomal RNA locus and development of a species-specific PCR-based diagnostic assay. The Journal of Eukaryotic Microbiology 48: 52-61.
Costa, P.M., S. Carreira, J. Lobo and M.H. Costa. 2012. Molecular detection of prokaryote and protozoan parasites in the commercial bivalve Ruditapes decussatus from southern Portugal. Aquaculture 370–371: 61-67.
Cremonte, F., P. Balseiro and A. Figueras. 2005. Occurrence of Perkinsus olseni (Protozoa: Apicomplexa) and other parasites in the venerid commercial clam Pitar rostrata from Uruguay, southwestern Atlantic coast. Diseases of Aquatic Organisms 64: 85-90.
Culurgioni, J., V. D'Amico, R. De Murtas, G. Canestri Trotti and V. Figus. 2006. Parasitological monitoring of commercial native bivalves from St. Gilla lagoon (Sardinia, South Western Mediterranean). Ittiopatologia 3: 243-252.
Dang, C., X. de Montaudouin, N. Caill-Milly and Z. Trumbiç. 2010. Spatio-temporal patterns of perkinsosis in the Manila clam Ruditapes philippinarum from Arcachon Bay (SW France). Diseases of Aquatic Organisms 91: 151-159.
Dang, C., X. de Montaudouin, C. Binias, F. Salvo, N. Caill-Milly, J. Bald and P. Soudant. 2013. Correlation between perkinsosis and growth in clams Ruditapes spp. Diseases of Aquatic Organisms 106: 255-265.
Da Ros, L. and W.J. Canzonier. 1985. Perkinsus, a protistan threat to bivalve culture in the Mediterranean basin. Bulletin of the European Association of Fish Pathologists 5: 23-25.
da Silva, P.M., H. Hégaret, C. Lambert, G.H. Wikfors, N. Le Goïc, S.E. Shumway and P. Soudant. 2008. Immunological responses of the Manila clam (Ruditapes philippinarum) with varying parasite (Perkinsus olseni) burden, during a long-term exposure to the harmful alga, Karenia selliformis, and possible interactions. Toxicon 51: 563-573.
De la Herrán, R., M.A. Garrido-Ramos, J.I. Navas, C. Ruiz Rejón and M. Ruiz Rejón. 2000. Molecular characterization of the ribosomal RNA gene region of Perkinsus atlanticus: its use in phylogenetic analysis and as a target for a molecular diagnosis. Parasitology 120: 345-353.
Dungan, C.F. and K.S. Reece. 2006. In vitro propagation of two Perkinsus spp. parasites from Japanese Manila clams Venerupis philippinarum and description of Perkinsus honshuensis n. sp. Journal of Eukaryotic Microbiology 53: 316-326.
Dungan, C.F., R.M. Hamilton, K.L. Hudson, C.B. McCollough and K.S. Reece. 2002. Two epizootic diseases in Chesapeake Bay commercial clams, Mya arenaria and Tagelus pledius. Diseases of Aquatic Organisms 50: 67-78.
Dungan, C.F., K.S. Reece, R.M. Hamilton, N.A. Stokes and E.M. Burreson. 2007a. Experimental cross-infection by Perkinsus marinus and P. chesapeaki in three sympatric species of Chesapeake Bay oysters and clams. Diseases of Aquatic Organisms 76: 67-75.
Dungan, C.F., K.S. Reece, J.A. Moss, R.M. Hamilton and B.K. Diggles. 2007b. Perkinsus olseni in vitro isolates from the New Zealand clam Austrovenus stutchburyi. Journal of Eukaryotic Microbiology 54: 263-270.
Elandalloussi, L.M., R. Afonso, P.A. Nunes and M.L. Cancela. 2003. Effect of desferrioxamine and 2,2'-bipyridyl on the proliferation of Perkinsus atlanticus. Biomolecular Engineering 20: 349-354.
Elandalloussi, L.M., R.M. Leite, R. Afonso, P.A. Nunes, J.A.F. Robledo, G.R. Vasta and M.L. Cancela. 2004. Development of a PCR-ELISA assay for diagnosis of Perkinsus marinus and Perkinsus atlanticus infections in bivalve molluscs. Molecular and Cellular Probes 18: 89-96.
Elandalloussi, L.M., R.B. Leite, P.M. Rodrigues, R. Afonso, P.A. Nunes and M.L. Cancela. 2005a. Effect of antiprotozoal drugs on the proliferation of the bivalve parasite Perkinsus olseni. Aquaculture 243: 9-17.
Elandalloussi, L.M., P.M. Rodrigues, R. Afonso, R.B. Leite, P.A. Nunes and M.L. Cancela. 2005b. Shikimate and folate pathways in the protozoan parasite, Perkinsus olseni. Molecular and Biochemical Parasitology 142: 106-109.
Elandaloussi, L.M., N. Carrasco, A. Roque, M. Fernández-Tejedor and D. Furones. 2008. Occurence of Perkinsus sp. in two clam species (Ruditapes philippinarum and R. decussatus) from the Ebro Delta, Spain. Bulletin of the European Association of Fish Pathologists 28: 1-9.
Elandaloussi, L.M., N. Carrasco, A. Roque, K. Andree and D.M. Furones. 2009a. First record of Perkinsus olseni, a protozoan parasite infecting the commercial clam Ruditapes decussatus in Spanish Mediterranean waters. Journal of Invertebrate Pathology 100: 50-53.
Elandaloussi, L., A. Carrasco, D. Furones and A. Roque. 2009b. Phylogenetic relationship of Perkinsus olseni from the Ebro Delta, Spain, to other Perkinsus species, based on ribosomal DNA sequences. Diseases of Aquatic Organisms 86: 135-142.
Elston, R.A., C.F. Dungan, T.R. Meyer and K.S. Reece. 2004. Perkinsus sp. infection risk for Manila clams, Venerupis philippinarum (A. Adams and Reeve, 1850) on the Pacific coast of North and Central America. Journal of Shellfish Research 22: 661-665 and Journal of Shellfish Research 23:101-105.
Feng, C., C. Wang, X. Lin, Y. Zhang, J. Lv, J.-h. Deng, X. Yuan, L. Mei and S.-q. Wu. 2013. Development of a loop-mediated isothermal amplification method for detection of Perkinsus spp. in mollusks. Diseases of Aquatic Organisms 104: 141-148.
Figueras, A., J.A.F. Robledo and B. Novoa. 1992. Occurrence of haplosporidian and Perkinsus-like infections in carpet-shell clams, Ruditapes decussatus (Linnaeus, 1758), of the Ria de Vigo (Galicia, NW Spain). Journal of Shellfish Research 11: 377-382.
Figueras, A., J.A.F. Robledo and B. Novoa. 1996. Brown ring disease and parasites in clams (Ruditapes decussatus and R. philippinarum) from Spain and Portugal. Journal of Shellfish Research 15: 363-368.
Figueras, A., G. Lorenzo, M.C. Ordás, M. Gouy and B. Novoa. 2000. Sequence of the small subunit ribosomal RNA gene of Perkinsus atlanticus-like isolated from carpet shell clam in Galicia, Spain. Marine Biotechnology 2: 419-428.
Figueras, A., C. Ordás, J. Gómez León and B. Novoa 2001. Molecular diagnosis of Perkinsus atlanticus and Pseudoperkinsus tapetis, parasites of the carpet shell clam (Ruditapes decussatus). Book of Abstracts, European Association of Fish Pathologists, Tenth International Conference "Diseases of Fish and Shellfish. Trinity College Dublin, Ireland, 9 - 14 September 2001. pg. 0-136. (Abstract).
Flassch, J.P. and Y. Leborgne. 1992. Introduction in Europe, from 1972 to 1980, of the Japanese Manila clam (Tapes philippinarum) and the effects on aquaculture production and natural settlement. International Council for Exploration of the Sea Marine Science Symposium 194: 92-96.
Goggin, C.L. 1994. Variation in the two internal transcribed spacers and 5.8S ribosomal RNA from five isolates of the marine parasite Perkinsus (Protista, Apicomplexa). Molecular and Biochemical Parasitology 65: 179-182.
Goggin, C.L. 1996. Effect of Perkinsus olseni (Protozoa, Apicomplexa) on the weight of Tridacna crocea (Mollusca, Bivalvia) from Lizard Island, Great Barrier Reef. Aquaculture 141: 25-30.
Goggin, C.L. and S.C. Barker. 1993. Phylogenetic position of the genus Perkinsus (Protista, Apicomplexa) based on small subunit ribosomal RNA. Molecular and Biochemical Parasitology 60: 65-70.
Goggin, C.L. and R.J.G. Lester. 1987. Occurrence of Perkinsus species (Protozoa, Apicomplexa) in bivalves from the Great Barrier Reef. Diseases of Aquatic Organisms 3: 113-117.
Goggin, C.L. and R.J.G. Lester. 1995. Perkinsus, a protistan parasite of abalone in Australia: a review. Marine Fisheries Research 46: 639-646.
Goggin, C.L., K.B. Sewell and R.J.G. Lester. 1989. Cross-infection experiments with Australian Perkinsus species. Diseases of Aquatic Organisms 7: 55-59.
Goggin, C.L., K.B. Sewell and R.J. Lester. 1990. Tolerances of Perkinsus spp. (Protozoa, Apicomplexa) to temperature, chlorine and salinity. Journal of Shellfish Research 9: 145-148.
Hamaguchi, M., N. Suzuki, H. Usuki and H. Ishioka. 1998. Perkinsus protozoan infection in short-necked clam Tapes (=Ruditapes) philippinarum in Japan. Fish Pathology (Tokyo) 33: 473-480.
Hégaret, H., P.M. da Silva, G.H. Wikfors, C. Lambert, T. De Bettignies, S.E. Shumway and P. Soudant. 2007. Hemocyte responses of Manila clams, Ruditapes philippinarum, with varying parasite, Perkinsus olseni, severity to toxic-algal exposures. Aquatic Toxicology 84: 469-479.
Hégaret, H., P.M. da Silva, I. Sunila, S.E. Shumway, M.S. Dixon, J. Alix, G.H. Wikfors and P. Soudant. 2009. Perkinsosis in the Manila clam Ruditapes philippinarum affects responses to the harmful-alga, Prorocentrum minimum. Journal of Experimental Marine Biology and Ecology 371: 112-120.
Hégaret, H., N. Henry, M. Bunel, M. Lassudrie, N. Le Goic, C. Lambert , A. Donval, C. Fabioux, X. De Montaudouin and P. Soudant. 2012. Impacts of Alexandrium ostenfeldii on behavioral and physiological responses of Manila clams Ruditapes philippinarum naturally infected with the parasite Perkinsus olseni. Journal of Shellfish Research 31: 295-296. (Abstract).
Hine, P.M. 2002. Results of a survey on shellfish health in New Zealand in 2000. Surveillance 29: 3-7.
Hine, P.M. and B.C. Diggles. 2002. The distribution of Perkinsus olseni in New Zealand bivalve molluscs. Surveillance 29: 8-11.
Hine, P.M. and T. Thorne. 2000. A survey of some parasites and diseases of several species of bivalve mollusc in northern Western Australia. Diseases of Aquatic Organisms 40: 67-78.
Kang, Y.-S., Y.-M. Kim, K.-I. Park, S.K. Cho, K.-S. Choi and M. Cho. 2006. Analysis of EST and lectin expressions in hemocytes of Manila clams (Ruditapes philippinarum) (Bivalvia: Mollusca) infected with Perkinsus olseni. Developmental and Comparative Immunology 30: 1119-1131.
Kang, Y.-S., K.S. Choi, Y.-B. Chung, S. Kim and M. Cho. 2008. Protease associated with lectin produced by Perkinsus olseni infected Manila clams (Ruditapes philippinarum). Bulletin of the European Association of Fish Pathologists 28: 89-96.
Kim, Y.M., K.-I. Park, K.-S. Choi, R.A. Alvarez, R.D. Cummings and M. Cho. 2006. Lectin from the Manila clam Ruditapes philippinarum is induced upon infection with the protozoan parasite Perkinsus olseni. The Journal of Biological Chemistry 281: 26854-26864.
Kleinschuster, S.J., F.O. Perkins, M.J. Dykstra and S.L. Swink. 1994. The in vitro life cycle of a Perkinsus species (Apicomplexa, Perkinsidae) isolated from Macoma balthica (Linneaus, 1758). Journal of Shellfish Research 13: 461-465.
Kotob, S.I., S.M. McLaughlin, P. van Berkum and M. Faisal. 1999a. Characterization of two Perkinsus spp. from the softshell clam, Mya arenaria using the small subunit ribosomal RNA gene. The Journal of Eukaryotic Microbiology 46: 439-444.
Kotob, S.I., S.M. McLaughlin, P. VanBerkum and M. Faisal. 1999b. Discrimination between two Perkinsus spp. isolated from the softshell clam, Mya arenaria, by sequence analysis of two internal transcribed spacer regions and the 5.8S ribosomal RNA gene. Parasitology 119: 363-368.
La Peyre, M., S. Casas and J. La Peyre. 2006. Salinity effects on viability, metabolic activity and proliferation of three Perkinsus species. Diseases of Aquatic Organisms 71: 59-74.
La Peyre, M.K., S.M. Casas, A. Villalba and J.F. La Peyre. 2008. Determination of the effects of temperature on viability, metabolic activity and proliferation of two Perkinsus species, and its significance to understanding seasonal cycles of perkinsosis. Parasitology 135: 505-519.
Lee, M.-K., B.-Y. Cho, S.-J. Lee, J.-Y. Kang, H.D. Jeong, S.H. Huh and M.-D. Huh. 2001. Histopathological lesions of Manila clam, Tapes philippinarum, from Hadong and Namhae coastal areas of Korea. Aquaculture 201: 199-209.
Leethochavalit, S., E.S. Upatham, K.-S. Choi, P. Sawangwong, K. Chalermwat and M. Kruatrachue. 2003. Ribosomal RNA characterization of non-transcribed spacer and two internal transcribed spacers with 5.8S ribosomal RNA of Perkinsus sp. found in undulated surf clams (Paphai undulata) from Thailand. Journal of Shellfish Research 22: 431-434.
Leethochavalit, S., K. Chalermwat, E.S. Upatham, K.S. Choi, P. Sawangwong and M. Kruatrachue. 2004. Occurrence of Perkinsus sp. in undulated surf clams Paphia undulata from the Gulf of Thailand. Diseases of Aquatic Organisms 60: 165-171.
Leite, R.B., R. Afonso and M.L. Cancela. 2004. Perkinsus sp. infestation in carpet-shell clams, Ruditapes decussatus (L), along the Portuguese coast. Results from a 2-year survey. Aquaculture 240: 39-53.
Liang, Y.-B., X.-C. Zhang, L.-J. Wang, B. Yang, Y. Zhang and C.-L. Cai. 2001. Prevalence of Perkinsus sp. in the Manila clam Ruditapes philippinarum along northern coast of Yellow Sea in China. Oceanologia et Limnologia Sinica 32: 502-511. (Chinese with English abstract.).
López, C., M.J. Carballal, C. Azevedo and A. Villalba. 1997. Differential phagocytic ability of the circulating haemocyte types of the carpet shell clam Ruditapes decussatus (Mollusca: Bivalvia). Diseases of Aquatic Organisms 30: 209-215.
Maeno, Y., T. Yoshinaga and K. Nakajima. 1999. Occurrence of Perkinsus species (Protozoa, Apicomplexa) from Manila clam Tapes philippinarum in Japan. Fish Pathology (Tokyo) 34:127-131.
Marques, A., J. Tato-Costa, C. Conde, C. Azevedo and M.L. Teles-Grilo. 2012. Chromosomal localisation of five genes in Perkinsus olseni (Phylum Perkinsozoa). European Journal of Protistology 48: 194-198.
McCoy, A., S.M. Baker and A.C. Wright. 2007. Investigation of Perkinsus spp. in aquacultured hard clams (Mercenaria mercenaria) from the Florida Gulf coast. Journal of Shellfish Research 26: 1029-1033.
McLaughlin, S.M. and M. Faisal. 1998a. Histopathological alterations associated with Perkinsus spp. infection in the softshell clam Mya arenaria. Parasite 5: 263-271.
McLaughlin, S.M. and M. Faisal. 1998b. In vitro propagation of two Perkinsus species from the softshell clam Mya arenaria. Parasite 5: 341-348.
McLaughlin, S.M. and M. Faisal. 1999. A comparison of diagnostic assays for detection of Perkinsus spp. in the softshell clam Mya arenaria. Aquaculture 172: 197-204.
McLaughlin, S.M. and M. Faisal. 2000. Prevalence of Perkinsus spp. in Chesapeake Bay soft-shell clams, Mya arenaria Linnaeus, 1758 during 1990-1998. Journal of Shellfish Research 19: 349-352.
McLaughlin, S.M. and M. Faisal. 2001. Pathogenesis of Perkinsus spp. in bivalve molluscs. Bulletin of the National Research Institute of Aquaculture Supplement 5: 111-117.
McLaughlin, S.M., E.E. Elsayed and M. Faisal. 2000a. Analysis of extracellular proteins of two Perkinsus spp. isolated from the softshell clam Mya arenaria in vitro. Comparative Biochemistry and Physiology Part B 126: 587-598.
McLaughlin, S.M., B.D. Tall, A. Shaheen, E.E. Elsayed and M. Faisal. 2000b. Zoosporulation of a new Perkinsus species isolated from the gills of the softshell clam Mya arenaria. Parasite 7: 115-122.
Miossec, L. 2006. Infection of two species of clams by Perkinsus olseni in France, DIPnet - Disease Interactions and Pathogen exchange between farmed and wild aquatic animal populations - a European network. Newsletter 43: 2 p.
Miossec, L., I. Arzul, C. Garcia, P. Soudant, C. Francois, R.B. Leite, M.L. Cancela and I. De Blas. 2005. Recommendations for evaluation of the health status in cultured and wild shellfish: Perkinsus olseni infestation in clams as an example. 8th International Conference on Shellfish Restoration (for electronic version of poster see: https://archimer.ifremer.fr/doc/00000/3326/).
Miossec, L., C. Garcia, I. Arzul, C. Francois, J.P. Joly, B. Chollet, M. Robert and I. De Blas. 2006. Infection of clams by Perkinsus olseni as an example of the new French Surveillance Programme. Proceedings of the 11th Symposium of the International Society for Veterinary Epidemiology and Economics, Cairns, Australia, Theme 1 - Aquatic animal epidemiology: Crustacean and shellfish disease session, August 2006, pp. 858-861 (for electronic versions see: https://archimer.ifremer.fr/doc/00000/6376/).
Montes, J.A., M. Durfort and J. García-Valero. 1996. When the venerid clam Tapes decussatus is parasitized by the protozoan Perkinsus sp. it synthesizes a defensive polypeptide that is closely related to p225. Diseases of Aquatic Organisms 26: 149-157.
Montes, J.F., J.A. Del Río, M. Durfort and J. García-Valero. 1997. The protozoan parasite Perkinsus atlanticus elicits a unique defensive response in the clam Tapes semidecussatus. Parasitology 114: 339-349.
Montes, J.F., M. Durfort and J. García-Valero. 2001. Parasitism by the protozoan Perkinsus atlanticus favours the development of opportunistic infections. Diseases of Aquatic Organisms 46: 57-66.
Montes, J.F., M. Durfort, A. Lladó and J. García-Valero. 2002. Characterization and immunolocalization of a main proteinaceous component of the cell wall of the protozoan parasite Perkinsus atlanticus. Parasitology 124: 477-484.
Moore, B.R., S.N. Kleeman and R.J.G. Lester. 2002. The development of a positive non-infectious control for the detection of Perkinsus using the Ray test. Journal of Shellfish Research 21: 871-873.
Mortensen, S., I. Arzul, L. Miossec, C. Paillard, S. Feist, G. Stentiford, T. Renault, D. Saulnier and A. Gregory. 2007. Molluscs and crustaceans, 5.3.18 Perkinsosis due to Perkinsus olseni. In: Raynard, R., T. Wahli, I. Vatsos, S. Mortensen (eds.) Review of disease interactions and pathogen exchange between farmed and wild finfish and shellfish in Europe. VESO on behalf of DIPNET, Oslo. p. 389-396.
Moss, J.A. and K.S. Reece. 2005. Pathogens of the non-native oyster, Crassostrea ariakensis, in its native range: current findings and work in progress. Journal of Shellfish Research 24: 668. (Abstract).
Moss, J.A., E.M. Burreson and K.S. Reece. 2006. Advanced Perkinsus marinus infections in Crassostrea ariakensis maintained under laboratory conditions. Journal of Shellfish Research 25: 65-72.
Moss, J.A., E.M. Burreson, J.F. Cordes, C.F. Dungan, G.D. Brown, A. Wang, X. Wu and K.S. Reece. 2007. Pathogens in Crassostrea ariakensis and other Asian oyster species: implications for non-native oyster introduction to Chesapeake Bay. Diseases of Aquatic Organisms 77: 207-223.
Moss, J.A., C.F. Dungan and K.S. Reece. 2008. Susceptibility of Chesapeake Bay bivalves to non-native Perkinsus species: pathogen risk associated with the introduction of Crassostrea ariakensis. Journal of Shellfish Research 27: 1034. (Abstract).
Murrell, A., S.N. Kleeman, S.C. Barker and R.J.G. Lester. 2002. Synonymy of Perkinsus olseni Lester & Davis, 1981 and Perkinsus atlanticus Azevedo, 1989 and an update on the phylogenetic position of the genus Prekinsus. Bulletin of the European Association of Fish Pathologists 22: 258-265.
Navas, J.I., M.C. Castillo, P. Vera and M. Ruiz-Rico. 1992. Principal parasites observed in clams, Ruditapes decussatus (L.), Ruditapes philippinarum (Adam et Reeve), Venerupis pullastra (Montagu) and Venerupis aureus (Gmelin), from the Huelva coast (S.W. Spain). Aquaculture 107: 193-199.
Nickens, A.D., J.F. La Peyre, E.S. Wagner and T.R. Tiersch. 2002. An improved procedure to count Perkinsus marinus in eastern oyster hemolymph. Journal of Shellfish Research 21: 725-732.
Ngo, T.T.T. and K.-S. Choi. 2004. Seasonal changes of Perkinsus and Cercaria infections in the Manila clam Ruditapes philippinarum from Jeju, Korea. Aquaculture 239: 57-68.
Novoa, B., M.C. Ordás and A. Figueras. 2002. Hypnospores detected by RFTM in clam (Ruditapes decussatus) tissues belong to two different protozoan organisms, Perkinsus atlanticus and a Perkinsus-like organism. Aquaculture 209: 11-18.
Ordás, M.C. and A. Figueras. 1998. In vitro culture of Perkinsus atlanticus, a parasite of the carpet shell clam Ruditapes decussatus. Diseases of Aquatic Organisms 33: 129-136.
Ordás, M.C., B. Novoa and A. Figueras. 1999. Phagocytosis inhibition of clam and mussel haemocytes by Perkinsus atlanticus secretion products. Fish and Shellfish Immunology 9: 491-503.
Ordás, M.C., A. Ordás, C. Beloso and A. Figueras. 2000. Immune parameters in carpet shell clams naturally infected with Perkinsus atlanticus. Fish and Shellfish Immunology 10: 597-609.
Ordás, M.C., J. Gomez-Leon and A. Figueras. 2001a. Histopathology of the infection by Perkinsus atlanticus in three clam species (Ruditapes decussatus, R. philippinarum and R. pullastra) from Galicia (NW Spain). Journal of Shellfish Research 20: 1019-1024.
Ordás, M.C., B. Novoa, M. Faisal, S. McLaughlin and A. Figueras. 2001. Proteolytic activity of cultured Pseudoperkinsus tapetis extracellular products. Comparative Biochemistry and Physiology 130B: 199-206.
Pardo, B.G., A. Cao, R. Vilas, E. Abollo, A. Villalba and P. Martínez. 2011. Microsatellite marker development in the protozoan parasite Perkinsus olseni. Diseases of Aquatic Organisms 94: 161-165.
Park, K.-I. and K.-S. Choi. 2001. Spatial distribution of the protozoan parasite Perkinsus sp. found in the Manila clams, Ruditapes philippinarum, in Korea. Aquaculture 203: 9-22.
Park, K.-I., K.-S. Choi and J.-W. Choi. 1999. Epizootiology of Perkinsus sp. found in the Manila clam, Ruditapes philippinarum in Komsoe Bay, Korea. Journal of the Korean Fisheries Society 32: 303-309.
Park, K.-I., K.-S. Choi and W.-G. Jeong. 2001. An examination for the protozoan parasite, Perkinsus sp. in the pearl oyster, Pinctada fucata martensii (Dunker) from the southern coast of Korea. Bulletin of the European Association of Fish Pathologists 21: 30-32.
Park, K.-I., J.-K. Park, J. Lee and K.-S. Choi. 2005. Use of molecular markers for species identification of Korean Perkinsus sp. isolated from Manila clams Ruditapes philippinarum. Diseases of Aquatic Organisms 66: 255-263.
Park, K.-I., T.T.T. Ngo, S.-D. Choi, M. Cho and K.-S. Choi. 2006a. Occurrence of Perkinsus olseni in the Venus clam Protothaca jedoensis in Korean waters. Journal of Invertebrate Pathology 93: 81-87.
Park, K.-I., A. Figueras and K.-S. Choi. 2006b. Application of enzyme-linked immunosorbent assay (ELISA) for the study of reproduction in the Manila clam Ruditapes philippinarum (Mollusca: Bivalvia): II. Impacts of Perkinsus olseni on clam reproduction. Aquaculture 251: 182-191.
Park, K.-I., H. Tsutsumi, J.-S. Hong and K.-S. Choi. 2008. Pathology survey of the short-neck clam Ruditapes philippinarum occurring on sandy tidal flats along the coast of Ariake Bay, Kyushu, Japan. Journal of Invertebrate Pathology 99: 212-219.
Park, K.-I., H.-S. Yang, H.-S. Kang, M. Cho, K.-J. Park and K.-S. Choi. 2010. Isolation and identification of Perkinsus olseni from feces and marine sediment using immunological and molecular techniques. Journal of Invertebrate Pathology 105: 261-269.
Pecher, W.T., J.A. Robledo and G.R. Vasta. 2004. Identification of a second rRNA gene unit in the Perkinsus andrewsi genome. Journal of Eukaryotic Microbiology 51: 234-245.
Pecher, W.T., M.R. Alavi, E.J. Schott, J.A. Fernandez-Robledo, L. Roth, S.T. Berg and G.R. Vasta. 2008. Assessment of the northern distribution range of selected Perkinsus species in eastern oysters (Crassostrea virginica) and hard clams (Mercenaria mercenaria) with the use of PCR-based detection assays. The Journal of Parasitology 95: 410-422.
Prado-Alvarez, M., C. Gestal, B. Novoa and A. Figueras. 2009. Differentially expressed genes of the carpet shell clam Ruditapes decussatus against Perkinsus olseni. Fish and Shellfish Immunology 26: 72-83.
Ramilo, A., J. Pintado, P. Ruíz, S. Darriba, A. Villalba and E. Abollo. 2011. Perkinsus spp. in clams Ruditapes decussatus, Ruditapes philippinarum, Venerupis senegalensis and Tapes rhomboides in Galicia (NW Spain): first detection of Perkinsus chesapeaki in Spain by DGGE. Journal of Shellfish Research 31: 336. (Abstract).
Ray, S.M. 1966. A review of the culture method for detecting Dermocystidium marinum, with suggested modifications and precautions. Proceedings of the National Shellfisheries Association 54: 55-69.
Reece, K.S., G.D. Brown, K.L. Hudson and K. Apakupakul. 2001. Inter- and intra-specific genetic variation among Perkinsus species: implications for species identification and development of molecular diagnostics. Journal of Shellfish Research 20: 554. (Abstract).
Reece, K.S., C.F. Dungan and E.M. Burreson. 2008. Molecular epizootiology of Perkinsus marinus and P. chesapeaki infections among wild oysters and clams in Chesapeake Bay, USA. Diseases of Aquatic Organisms 82: 237-248.
Robledo, J.A.F., C.A. Coss and G.R. Vasta. 1999. Development of a PCR-based diagnostic assay from a novel Perkinsus species isolated from Macoma baltica. Journal of Shellfish Research 18: 333. (Abstract).
Robledo, J.A.F., C.A. Coss and G.R. Vasta. 2000. Characterization of the ribosomal RNA locus of Perkinsus atlanticus and development of a polymerase chain reaction-based diagnostic assay. The Journal of Parasitology 86: 972-978.
Robledo, J.A.F., P.A. Nunes, M.L. Cancela and G.R. Vasta. 2002. Development of an in vitro clonal culture and characterization of the rRNA gene cluster of Perkinsus atlanticus, a protistan parasite of the clam Tapes decussatus. Journal of Eukaryotic Microbiology 49: 414-422.
Rodríguez, F. and J.I. Navas. 1995. A comparison of gill and hemolymph assays for the thioglycollate diagnosis of Perkinsus atlanticus (Apicomplexa, Perkinsea) in clams, Ruditapes decussatus (L.) and Ruditapes philippinarum (Adams et Reeve). Aquaculture 132: 145-152.
Rodríguez, F., T. Godoy and J.I. Navas. 1994. Cross-infection with Perkinsus atlanticus in Ruditapes decussatus, Ruditapes philippinarum and Venerupis pullastra. Bulletin of the European Association of Fish Pathologists 14: 24-27.
Sagristà, E., M. Durfort and C. Azevedo. 1995. Perkinsus sp. (Phylum Apicomplexa) in Mediterranean clam Ruditapes semidecussatus: ultrastructural observations of the cellular response of the host. Aquaculture 132: 153-160.
Sagristà, E., M. Durfort and C. Azevado. 1996. Ultrastructural data on the life cycle of the parasite, Perkinsus atlanticus (Apicomplexa), on the clam, Ruditapes philippinarum, in the Mediterranean. Scientia Marina 60: 283-288.
Sheppard, B.J. and C.F. Dungan. 2009. Exotic Perkinsus sp. protozoa in an imported Vietnamese ornamental clam (Tridacna crocea) maintained in a home aquarium. Journal of Zoo and Wildlife Medicine 40: 140-146.
Shimokawa, J., T. Yoshinaga and K. Ogawa. 2010. Experimental evaluation of the pathogenicity of Perkinsus olseni in juvenile Manila clams Ruditapes philippinarum. Journal of Invertebrate Pathology 105: 347-351.
Teles-Grilo, M.L., J. Tato-Costa, S.M. Duarte, A. Maia, G. Casal and C. Azevado. 2007. Is there a plastid in Perkinsus atlanticus (Phylum Perkinsozoa)? European Journal of Protistology 43: 163-167.
Teles-Grilo, M.L., S.M. Duarte, J. Tato-Costa, A. Gaspar-Maia, C. Oliveira, A.A. Rocha, A. Marques, A. Cordeiro-da-Silva and C. Azevado. 2007. Molecular karyotype analysis of Perkinsus atlanticus (Phylum Perkinsozoa) by pulsed field gel electrophoresis. European Journal of Protistology 43: 315-318.
Valiulis, G.A. and J.G. Mackin. 1969. Formation of sporangia and zoospores by Labyrinthomyxa sp. parasitic in the clam Macoma balthica. Journal of Invertebrate Pathology 14: 268-270.
Villalba, A. and S.M. Casas. 2001. Effect of perkinsosis on the energetic physiology of the clam Ruditapes decussatus. Journal of Shellfish Research 20: 559-560. (Abstract).
Villalba, A. and J.I. Navas. 1988. Occurrence of Minchinia tapetis and a Perkinsus-like parasite in cultured clams, Ruditapes decussatus and R. philippinarum, from south Atlantic coast of Spain. Preliminary results. In: F.O. Perkins and T.C. Cheng (eds.). Abstracts, Third International Colloquium on Pathology in Marine Aquaculture, 2-6 Oct. 1988. Gloucester Point, VA. Virginia Institute of Marine Science, Gloucester Point, p. 57-58.
Villalba, A., S.M. Casas, M.J. Carballal and C. López. 2000. Effects of perkinsosis on the clam Ruditapes decussatus industry of Galicia (NW Spain). Journal of Shellfish Research 19: 649. (Abstract).
Villalba, A., K.S. Reece, M.C. Ordás, S.M. Casas and A. Figueras. 2004. Perkinsosis in molluscs: A review. Aquatic Living Resources 17: 411-432.
Villalba, A., S.M. Casas, C. López and M.J. Carballal. 2005. Study of perkinsosis in the carpet shell clam Tapes decussatus in Galicia (NW Spain). II. Temporal pattern of disease dynamics and association with clam mortality. Diseases of Aquatic Organisms 65: 257-267.
Wu, S.-q., C.-x. Wang, X.-m. Lin, Z.-x. Wang, X.-f. Li, J. Liu, J.-h. Deng and S.-y. Qiu. 2011. Infection prevalence and phylogenetic analysis of Perkinsus olseni in Ruditapes philippinarum from East China. Diseases of Aquatic Organisms 96: 55-60.
Yang, H.-S., K.-I. Park, L. Donaghy, M. Adhya and K.-S. Choi. 2012. Temporal variation of Perkinsus olseni infection intensity in the Manila clam Ruditapes philippinarum in Gomso Bay, off the west coast of Korea. Journal of Shellfish Research 31: 685-690.
Yoshinaga, T., S. Watanabe, T. Waki, S. Aoki and K. Ogawa. 2010. Influence of Perkinsus infection on the physiology and behavior of adult Manila clam Ruditapes philippinarum. Fish Pathology 45: 151-157.
Zhang, H., D.A. Campbell, N.R. Sturm, C.F. Dungan and S. Lin. 2011. Spliced leader RNAs, mitochondrial gene frameshifts and multi-protein phylogeny expand support for the genus perkinsus as a unique group of alveolates. PLoS ONE 6(5): e19933. doi:19910.11371/journal.pone.0019933 (for electronic version see: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0019933).
Citation Information
Bower, S.M. (2013): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Perkinsus of Clams and Cockles.
Date last revised: December 2013
Comments to Susan Bower
- Date modified: