Kidney Coccidia of Clams
On this page
Category
Category 2 (In Canada and of Regional Concern)
Common, generally accepted names of the organism or disease agent
Clam kidney coccidia.
Scientific name or taxonomic affiliation
- Pseudoklossia glomerata of the family Aggregatidae Labbé.
- Pseudoklossia (= Hyaklossia) pelseneeri of the family Aggregatidae Labbé.
- Pseudoklossia sp.
- Margolisiella (= Klossia) (=Pseudoklossia) (=Merocystis) tellinae of the family Eimeriidae Minchin.
- Margolisiella kabatai of the family Eimeriidae Minchin.
- Unidentified species.
Other species of coccidia have been described from the kidneys of oysters, mussels, scallops and abalone from various locations around the world.
Geographic distribution
- Mediterranean Sea.
- France
- Galicia, Spain.
- Scotland.
- British Columbia, Canada and Washington, USA.
- German North Sea coast
Host species
- Tapes floridus, Tapes virgineus.
- Tellina sp., Donax sp.
- Cerastoderma edule.
- Tellina tenuis.
- Protothaca staminea.
- Scrobicularia plana
Impact on the host
Infected kidney epithelial cells become hypertrophied and the kidney tubules fill with coccidia. Heavy infections have been observed to spread to other tissues. Heavy infections may cause kidney damage but associated mortalities appear restricted to artificial growing conditions. The unidentified species in Scrobicularia plana has been observed to cause severe necrosis and chronic accumulation of haemocytes containing brownish pigment and in some cases the mid-gut (digestive) gland appeared to be involved (personal communication from B. T. Watermann, e-mail: mail@limnomar). Species in the family Eimeriidae are homoxenous (entire life cycle completed in one host) and thus could produce serious disease in clams being cultured in a closed system.
Diagnostic techniques
Squash Preparations
Preliminary diagnosis can be made on the presence of large mature macrogamonts (about 20 to 40 µm in diameter) in squashes of kidney tissues.
Histology
Various stages of the coccidia can be observed in the cytoplasm of kidney cells, in the kidney tubule lumens and occasionally within the connective tissue of the digestive gland, gills, gonads, mantle, etc. The following figures are all of an Eimeriidae, M. kabatai, in wild populations of P. staminea. The mature oocysts of M. kabatai were spherical (41 µm (range 30-44) in diameter) and contained about 32 subspherical sporocysts (9 x 10 µm), each of which contained 4 sporozoites. Spherical 19 µm (range 18-20) cyst-like structures, smaller multinucleated bodies (some of which resembled sporocysts) and all other life stages typical of the Eimeriidae (i.e., merogonic, gamontogonic and sporogonic developmental stages) were present in the renal tissue of infected clams.
Figures 1 to 8. Histological sections (haematoxylin and eosin stained) of renal tissue of Protothaca staminea infected with various stages of Margolisiella kabatai.
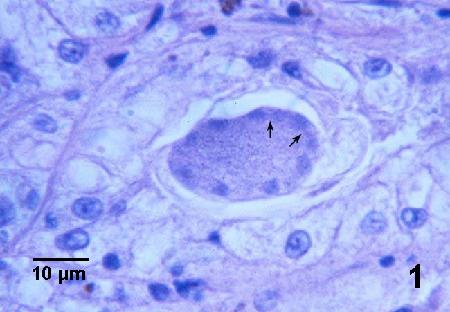
Figure 1. An immature meront containing peripherally located nuclei, two of which appear to be dividing (arrows).

Figure 2. Early development of merozoites (arrows) along the periphery of the meront.
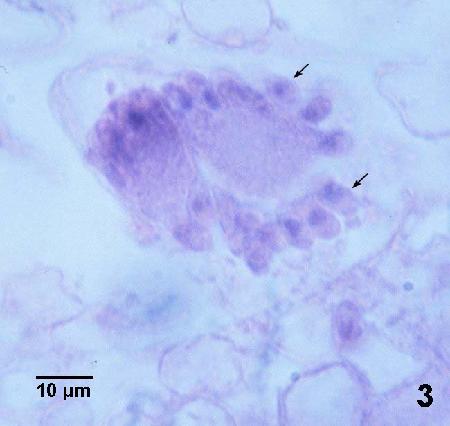
Figure 3. Merozoites (arrows) budding from the surface of a maturing meront.
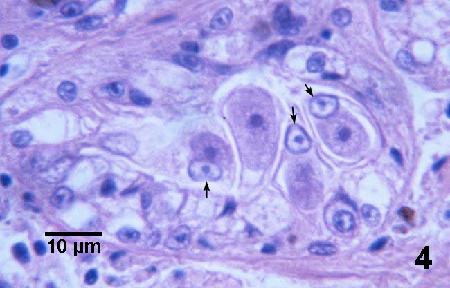
Figure 4. Four adjacent trophozoites each within the cytoplasm of an epithelial cell. Note the hypertrophied nucleus (arrows) of each infected cell and that the cell has expanded to accommodate the relatively large parasite.
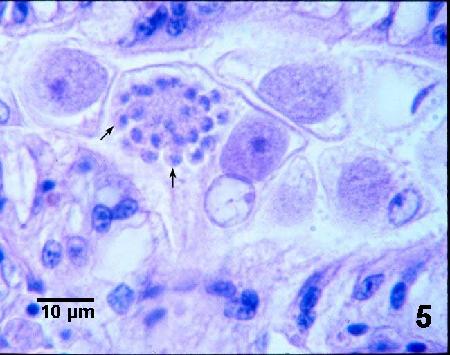
Figure 5. A mature microgamont with peripherally arranged microgametes (arrows) and a trophozoite in the cytoplasm of a renal epithelial cell.
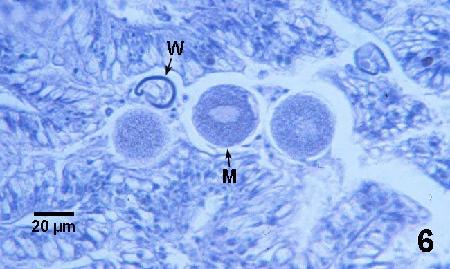
Figure 6. Mature macrogamonts (M) and a thick wall (W) that may be from an empty cyst-like stage of M. kabatai.

Figure 7. Glancing section through a young oocyst with multiple sporoblast nuclei. As in other infected renal epithelial cells, the nucleus (HN) of this host cell is hypertrophied.

Figure 8. Small multinucleated bodies within the lumen of a kidney tubule. Note that about eight nuclei can be seen in one body (arrow).
Images of the kidney coccidia in Scrobicularia plana as provided by B. T. Watermann (2001) are as follows:
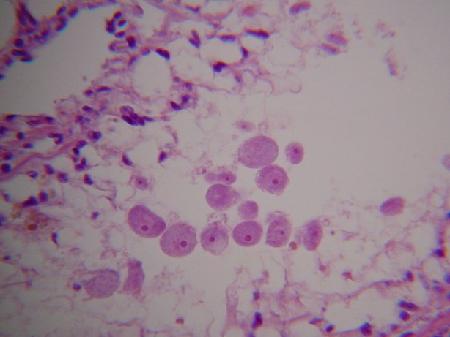
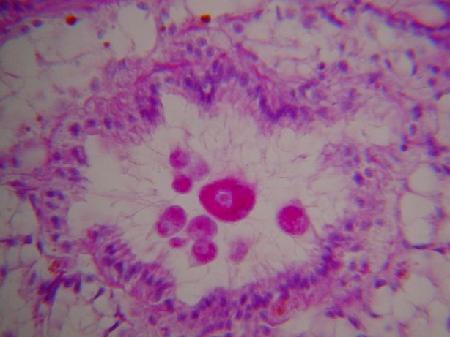
Figures 9 and 10. Macrogamonts within the lumen of kidney tubules.
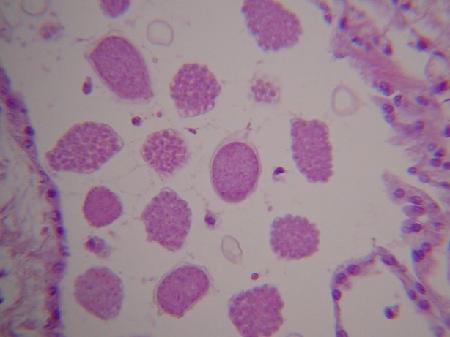

Figures 11 and 12. Various developmental stages in the lumen of kidney tubules.
Methods of control
No known methods of prevention or control. The species in the family Aggregatidae are heteroxenous and thus require another host (although none have been yet described) to complete the life cycle. Once the entire life cycle of these parasites are understood, management techniques may be feasible to eliminate the other host(s) and thus the parasite from the culture facility.
References
Bower, S.M. (2007): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Kidney Coccidia of Clams.
Lauckner, G. 1983. Diseases of Mollusca: Bivalvia. In: O. Kinne (ed.). Diseases of Marine Animals. Volume II: Introduction, Bivalvia to Scaphopoda. Biologische Anstalt Helgoland, Hamburg, p. 548-549.
Morado, J.F., A.K. Sparks and S.K. Reed. 1984. A coccidian infection of the kidney of the native littleneck clam Protothaca staminea. Journal of Invertebrate Pathology 43: 207-217.
Watermann, B.T. 2001. For further details concerning the coccidia in Scrobicularia plana, please contact Dr. Burkard Watermann at: LimnoMar, Bei der Neuen Münze 11, 22145 Hamburg, Germany. Phone: + 49 - 40 - 678 99 11, Fax: + 49 - 40 - 679 92 04, E-Mail: watermann@limnomar.de, Web site: http://www.limnomar.de
Citation Information
Bower, S.M. (2007): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Kidney Coccidia of Clams.
Date last revised: October 2007
Comments to Susan Bower
- Date modified: