Apicomplexan Parasite of New Zealand Oysters
On this page
Category
Category 3 (Host not in Canada)
Common, generally accepted names of the organism or disease agent
Apicomplexan infection of New Zealand oysters, flat oyster coccidiosis.
Scientific name or taxonomic affiliation
Unnamed apicomplexan parasite that does not resemble other apicomplexans reported from molluscs (Hine 2002). The only stage observed were called zoites because it is not known if they arise by sporogony or merogony and ultrastructural features that distinguish between merozoites, tachyzoites, bradyzoites and sporozoites of apicomplexans are lacking (Hine 2002).
Geographic distribution
Common in Ostrea chilensis all around New Zealand usually at low intensity but high prevalences and especially noted from populations around the South Island, in the Chatham Islands, in Wellington harbour, and around the Coromandel Peninsula.
Host species
Ostrea (=Tiostrea) chilensis.
Impact on the host
The apicomplexan had a considerable impact on some O. chilensis populations in New Zealand. Prevalence is often high - between 1986 and 1991 it was reported to range from 85% and 99% in over 6400 O. chilensis from Foveaux Strait where heavy infections resulted in massive damage to the vesicular connective tissue, host sterility and death (Hine and Jones 1994, Hine 2002). There was no discernable patterns in seasonal prevalence or intensity. The life cycle of this parasite is unknown. However, the occurrence of only one stage in the tissues of O. chilensis suggests that the parasite is heteroxenous. Hine (2002) suggested that other stages of this oyster pathogen may be the sexual stages of the apicomplexan detected in the gut epithelium of the terebellid polychaete Pseudopista rostrata, that lives in close contact with the oysters. However, confirmatory diagnosis using such techniques as molecular assays are required.
Although Hine (1991) initially proposed that the intensity of the apicomplexan infection did not affect either the intensity or distribution of concurrent Bonamia exitiosa (=exitiosus) infections, subsequent information and analysis suggested that zoites may play an important role in B. exitiosa pathogenicity (Hine 2002). The prevalence and intensity of B. exitiosa was related to the intensity of the apicomplexan zoites but the converse was not the case suggesting that the zoites may increase the sensitivity of the oysters to B. exitiosa by occupying and destroying haemocytes, by destroying connective tissue cells and by utilising host glycogen reserves (Hine 2002). Also, other stressors such as exposure to extreme temperatures (7 or 26°C) and salinity (40 ‰), starvation (prolonged holding in filtered sea water), or handling (vigorous stirring four time per day) can affect the dynamics of B. exitiosa in O. chilensis (Hine et al.2002).
Diagnostic techniques
Histology
The parasite is often found in haemolymph sinuses (possibly in haemocytes) in the suprabranchial sinuses, kidneys and between the digestive gland tubules. In heavy infections, it is also occurred in the vesicular connective (Leydig) tissue resulting in extensive tissue damage and may occur within (intracellular) the vesicular connective tissue cells. Lesions contained many haemocytes phagocytosing zoites, leading to haemocyte lysis and caused haemocytosis (Hine 2002). Intraepithelial infections of the kidney and gonad were also noted (Hine and Jones 1994).
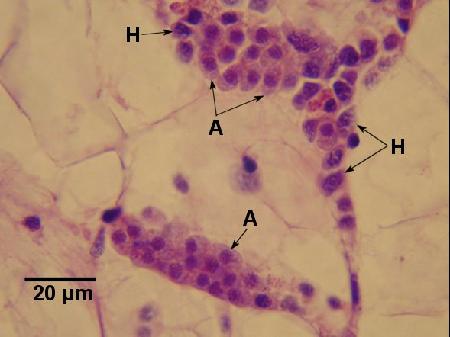
Figure 1. Two clusters of apicomplexa (A) and a few haemocytes (H) between vesicular connective tissue cells of Ostrea chilensis. Image provided by Ben Diggles PhD, DigsFish Services, www.digsfish.com, ben@digsfish.com.

Figure 2. Many apicomplexa (A) interspersed with haemocytes (H) in the connective tissue of Ostrea chilensis. The cytoplasm of the apicomplexa is slightly more basophilic and has a frothy appearance in comparison to that of the haemocytes. Note the Bonamia exitiosa (B) in one of the haemocytes. Image provided by Ben Diggles PhD, DigsFish Services, www.digsfish.com, ben@digsfish.com.
Electron Microscopy
The zoites contained all the structures typical of apicomplexan zoites including two polar rings, 84 sub-pellicular microtubules, a conoid, rhoptries and micronemes. The zoites were elongated and elliptical (about 8 µm long and 5 µm wide) with a round nucleus occupying almost the entire width of the cell halfway down its length (Hine 2002).
Methods of control
No known methods of prevention or control.
References
Hine, P.M. 1991. The annual pattern of infection by Bonamia sp. in New Zealand flat oysters, Tiostrea chilensis. Aquaculture 93: 241-251.
Hine, P.M. 2002. Severe apicomplexan infection in the oyster Ostrea chilensis: a possible predisposing factor in bonamiosis. Diseases of Aquatic Organisms 51: 49-60.
Hine, P.M. and J.B. Jones. 1994. Bonamia and other aquatic parasites of importance to New Zealand. New Zealand Journal of Zoology 21: 49-56.
Hine, P.M., B.K. Diggles, M.J.D. Parsons, A. Pringle and B. Bull. 2002. The effects of stressors on the dynamics of Bonamia exitiosus Hine, Cochennec-Laureau & Berthe, infections in flat oysters Ostrea chilensis (Philippi). Journal of Fish Diseases 25: 545-554.
Citation Information
Bower, S.M. (2006): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Apicomplexan Parasite of New Zealand Oysters
Date last revised: December 2006
Comments to Susan Bower
- Date modified: